Abstract
Intestinal oxidative stress triggers gut microbiota dysbiosis, which is involved in the etiology of post-weaning diarrhea and enteric infections. Ellagic acid (EA) can potentially serve as an antioxidant supplement to facilitate weaning transition by improving intestinal oxidative stress and gut microbiota dysbiosis. Therefore, we aimed to investigate the effects of dietary EA supplementation on the attenuation of intestinal damage, oxidative stress, and dysbiosis of gut microbiota in weanling piglets. A total of 126 piglets were randomly assigned into 3 groups and treated with a basal diet and 2 mL saline orally (Ctrl group), or the basal diet supplemented with 0.1% EA and 2 mL saline orally (EA group), or the basal diet and 2 mL fecal microbiota suspension from the EA group orally (FEA group), respectively, for 14 d. Compared with the Ctrl group, EA group improved growth performance by increasing average daily feed intake and average daily weight gain (P < 0.05) and decreasing fecal scores (P < 0.05). EA group also alleviated intestinal damage by increasing the tight junction protein occludin (P < 0.05), villus height, and villus height-to-crypt depth ratio (P < 0.05), while decreasing intestinal epithelial apoptosis (P < 0.05). Additionally, EA group enhanced the jejunum antioxidant capacity by increasing the total antioxidant capacity (P < 0.01), catalase (P < 0.05), and glutathione/oxidized glutathione (P < 0.05), but decreased the oxidative metabolite malondialdehyde (P < 0.05) compared to the Ctrl group. Compared with the Ctrl group, EA and FEA groups increased alpha diversity (P < 0.05), enriched beneficial bacteria (Ruminococcaceae and Clostridium ramosum), and increased metabolites short-chain fatty acids (P < 0.05). Correspondingly, FEA group gained effects comparable to those of EA group on growth performance, intestinal damage, and intestinal antioxidant capacity. In addition, the relative abundance of bacteria shifted in EA and FEA groups was significantly related to the examined indices (P < 0.05). Overall, dietary EA supplementation could improve growth performance and attenuate intestinal damage and oxidative stress by regulating the gut microbiota in weanling piglets.
Keywords: Ellagic acid, Gut microbiota, Weanling piglets, Intestinal damage, Oxidative stress
Highlights
-
•
Dietary supplementation with 0.1% EA significantly improved diarrhea, growth performance, intestinal damage, gut microbial dysbiosis, and intestinal antioxidative capacity.
-
•
Antioxidative agents EA can be used as antibiotics alternatives to improve weaning stress in piglets by restoring a balanced gut microbiota.
1. Introduction
The weaning age of piglets is approximately 3 wk in intensive swine production while the natural weaning age is approximately 17 wk (Jensen, 1986). The early weaning of piglets is frequently accompanied by transient anorexia, severe intestinal damage, infections, and diarrhea due to sudden changes in diet, social relationships, and the environment (Campbell et al., 2013; Lallès et al., 2007b). These multiple issues encountered in piglets during early weaning led to the overuse of antibiotics and dietary supplementation of zinc and copper beyond nutritional requirements, burdening the environment and public health (Gresse et al., 2017; Yazdankhah et al., 2014). Europe has introduced legislation to limit the amount of zinc oxide added to feed to less than 150 mg/kg (Starke et al., 2014). With the expansion of the ban on antibiotics for swine disease, growth promotion in China, and restrictions on copper and zinc additions, the search for antibiotic alternatives has become urgent (Allen et al., 2013, 2014; Gresse et al., 2017; Walsh and Wu, 2016). Increasing evidences indicate that early weaning stress leads to dysbiosis of gut microbiota, which is involved in the etiology of diarrhea and enteric infections in piglets (Gresse et al., 2017; Lallès et al., 2007a). Therefore, nonantibiotic functional additives that could restore a balanced gut microbiota are potentially effective agents that contribute to the weaning transition of piglets (Gresse et al., 2017).
Oxidative stress in the inflamed gut lumen disturbs the oxygen-sensitive niches where microbiota resides, thus triggering dysbiosis of the gut microbiota (Donaldson et al., 2016; Gresse et al., 2017). The gut microbiota shift effect of polyphenols has been found to have various pharmacological effects, including anti-neurodegenerative diseases (Nargeh et al., 2021), anti-nonalcoholic fatty liver disease (Wang et al., 2021), and alleviation of colitis (Zhao and Jiang, 2021). Furthermore, the gut microbiota of green tea polyphenol-treated mice improved intestinal epithelial homeostasis and ameliorated experimental colitis, indicating that gut microbiota mediates the function of polyphenols (Wu et al., 2021). Ellagic acid (EA), a polyphenol from several fruits and Chinese herbs, has excellent antioxidant capacity (Cornélio Favarin et al., 2013). The protective effects of EA against alcoholic liver disease in mice are associated with the gut microbiota (Zhao et al., 2021). Here, we aimed to investigate the effects of dietary EA supplementation on the attenuation of intestinal damage, oxidative stress, and dysbiosis of gut microbiota in weanling piglets. Fecal microbiota transplantation (FMT) provides an effective way to reveal the role of the gut microbiota in the pharmacodynamic effects of natural active ingredients (Dong et al., 2021; Wu et al., 2019). To test our hypothesis, we treated weanling piglets with EA and transferred their fecal microbiota to FMT recipient piglets.
2. Materials and methods
2.1. Animal ethics statement
Our animal trial was performed in accordance with the protocol approved by the Scientific Ethics Committee of Huazhong Agricultural University (approval number HZAUSW20210012).
2.2. Animals and experimental treatments
We allotted 126 early weaning Landrace × Yorkshire piglets (23 ± 1 d of age) into 3 groups of 7 replicates (6 piglets per replicate) and treated them with basal diet and 2 mL saline orally every other day (Ctrl group), or basal diet supplemented with 0.1% EA (content 82%, Tianxin Biotech Co., Ltd., Hubei, China) and 2 mL saline orally every other day (EA group), or basal diet and 2 mL fecal microbiota suspension from EA group orally every other day (FEA group), respectively, for 14 d. Then, 20 piglets were sacrificed for sampling (6 from the Ctrl group; 7 from the EA group, and 7 from the FEA group). The composition of the basal experimental diet (Table 1) is in accordance with the recommendations of the National Research Standards Committee (NRC, 2012). The dose of dietary EA supplementation was based on the literature (Cornélio Cornélio Favarin et al., 2013) and the results of our prior pre-trial (Fig. S1).
Table 1.
Ingredients and nutrients levels of basal diets (as-fed basis, %).
| Item | Content |
|---|---|
| Ingredients | |
| Corn | 20.02 |
| Soybean meal | 8 |
| Expanded soy bran | 10 |
| Expanded corn | 35.00 |
| Fermented soybean meal | 7.00 |
| Soybean oil | 1 |
| Sucrose | 3.00 |
| Intestinal membrane protein powder (50% CP) | 3.00 |
| Low protein whey powder | 6.00 |
| Fish meal | 3.00 |
| L-Lysine HCl | 0.45 |
| DL-Methionine | 0.15 |
| L-Threonine | 0.15 |
| L-Tryptophan | 0.03 |
| Choline chloride | 0.10 |
| Limestone | 0.90 |
| Dicalcium phosphate | 0.90 |
| NaCl | 0.30 |
| Vitamin and mineral premix1 | 1 |
| Nutrient levels | |
| DM | 88.57 |
| DE, MJ/kg | 14.35 |
| CP | 19.01 |
| Ca | 0.81 |
| Total P | 0.58 |
| Available P | 0.42 |
| Lysine | 1.41 |
| Methionine | 0.46 |
| Methionine + Cystine | 0.73 |
| Threonine | 0.87 |
| Tryptophan | 0.25 |
Provided per kilogram of complete diet; vitamin A: 1,5500 IU; vitamin D3: 3,000 IU; vitamin E: 40.0 mg; vitamin K: 1 mg; vitamin B1: 4.5 mg; vitamin B2:10.5 mg; vitamin B6: 7 mg; vitamin B12: 0.04 mg; folic acid: 2.0 mg; nicotinamide: 45 mg; D-biotin: 0.3 mg; D-pantothenic acid: 25 mg; Fe (as FeSO4•H2O), 100 mg; Cu (as CuSO4•5H2O), 6 mg; Mn (as MnSO4•5H2O), 20 mg; Zn (as ZnSO4•7H2O), 90 mg; I (as KI), 0.14 mg; Se (as Na2SeO3•5H2O), 0.30 mg.
2.3. Blood and intestinal sample collection
We obtained the blood samples using heparinized vacutainer tubes and then centrifuged them at 4 °C, 3,000 × g for 10 min. Three 3-cm long segments of jejunum tissue (Prates et al., 2021; Qiu et al., 2021) were sampled from the middle portion of small intestine and then rinsed with ice cold PBS. One segment was fixed in 4% paraformaldehyde for analyzing intestinal morphology, and the others were frozen immediately with dry ice. The feces samples were collected from rectum and then immediately frozen with dry ice. All frozen samples were then stored at −80 °C for the next analysis.
2.4. Piglet growth performance and fecal scores
We determined piglets’ average daily gain (ADG), average daily feed intake (ADFI) and fecal scores with the following scale: 1 = normal, solid feces; 2 = soft, looser than normal feces, slight diarrhea; 3 = moderate diarrheic feces; 4 = liquid, severe diarrheic feces for all pigs daily (Yi et al., 2005).
2.5. Histomorphology examination of the jejunum tissue
Jejunum tissues were fixed with 4% paraformaldehyde and then packed with paraffin wax. Consecutive sections at 5 mm thickness were stained with hematoxylin and eosin (H&E) for histomorphology examination. We determined villus height, crypt depth, and villus height-to-crypt depth ratio of the jejunum tissue at 40× magnification using a microscope (BX51, OLYMPUS, Japan).
2.6. Apoptosis assessment of jejunal epithelium
We determined apoptosis of jejunal epithelium using the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay (Gavrieli et al., 1992). The average optical density (AOD) of TUNEL (green) was determined using Image J to determine the apoptosis level of jejunal epithelium (Schneider et al., 2012).
2.7. Antioxidant indices determination of the jejunum tissue
We determined the antioxidant indices of total antioxidant capacity (T-AOC, BC1315), glutathione (GSH, BC1175)/oxidized glutathione (GSSG, BC1185), malondialdehyde (MDA, BC0025), catalase (CAT, BC0205), and nitric oxide (NO, BC1475) in jejunum tissue according to the manufacturer's instruction of commercial kits (Solarbio Science & Technology, Beijing, China). For tissue homogenates, 100 mg tissue was rinsed with PBS, homogenized in 1 mL of PBS and the homogenates were centrifuged for 5 min at 5,000 × g, and 4 °C. The supernatant was removed and assayed immediately.
2.8. Tight junction protein determination of the jejunum tissue
The jejunum tissues were suspended and homogenized in RIPA buffer containing protease inhibitors using a Tissuelyser (65 Hz for 120 s). After centrifugation at 12,000 × g, 4 °C for 15 min, the supernatants were collected and their protein concentrations were determined using BCA kit (Thermo Scientific, 23250). An equivalent amount of protein (12 μg) was separated by polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane. After TBST cleaning and sealing with 5% skim milk, the membrane was incubated with rabbit polyclonal antibodies occludin (Cell Signaling Technology, 91131S, 1:1,000) and claudin-1 (Proteintech, 13050-1-AP, 1:1,000) at 4 °C overnight. The membrane was then incubated with secondary antibody (1:10,000) at room temperature for 1.5 h. Bands were measured by densitometry using image J software and relative protein expression levels were standardized with β-actin.
2.9. The mRNA expression of inflammation- and apoptosis-related genes in jejunum tissue
Total RNA was extracted from jejunum tissues by TRIzol (Invitrogen). The concentration of total RNA was determined by a spectrophotometer (NanoDrop 2000, Thermo Scientific), and 2 mg total RNA was used for cDNA synthesis with reverse transcription master mix (Thermo Fisher Scientific) according to the manufacturer's instructions. The qPCR (95 °C for 5 min; 40 cycles of 95 °C for 15 s, 60 °C for 30 s and 72 °C for 30 s, and a final extension of 72 °C) was performed using the iTaq Universal SYBR Green Super Mix (Bio-Rad) on a CFX384 Real-Time PCR system (Bio-Rad) and the gene expression were normalized to GAPDH. All RT-qPCR primer pairs are listed in Table 3.
Table 3.
Primers sequences of target genes selected for analysis by real-time.
| Gene | Primer sequences (5′-3′) | Size, bp | Temperature, °C |
|---|---|---|---|
| TNF-α | F: ACCTCCTCTCTGCCATCAAG R: CTGCCCAGATTCAGCAAAGT |
173 | 60 |
| IL-1β | F: ATTCAGGGACCCTACCCTCTC R: ATCACTTCCTTGGCGGGTTC |
92 | 60 |
| IL-1α | F: GCATGTGCTGAGCCTTTGTA R: CCTGGTCCTCCCAAGATTGT |
181 | 60 |
| IL-6 | F: GATGCTTCCAATCTGGGTTCA | 62 | 58 |
| R: CACAAGACCGGTGGTGATTC | |||
| IL-10 | F: CGGCCCAGTGAAGAGTTTCT | 98 | 60 |
| R: GGCAACCCAGGTAACCCTTA | |||
| SOD | F: GAGACCTGGGCAATGTGACT | 118 | 60 |
| R: CTGCCCAAGTCATCTGGTTT | |||
| GCLC | F: CAAACCATCCTACCCTTTGG | 172 | 58 |
| R: ATTGTGCAGAGAGCCTGGTT | |||
| GCLM | F: GATGCCGCCCGATTTAACTG | 159 | 58 |
| R: ACAATGACCGAGTACCGCAG | |||
| HO-1 | F: CGCTCCCGAATGAACACTCT | 148 | 60 |
| R: GCGAGGGTCTCTGGTCCTTA | |||
| NQO-1 | F: ATCACAGGTAAACTGAAGGACCC | 229 | 60 |
| R: TGGCAGCGTATGTGTAAGCA | |||
| β-Actin | F: TCTGGCACCACACCTTCT R: TGATCTGGGTCATCTTCTCAC |
114 | 57 |
TNF-α = tumor necrosis factor-α; IL = interleukin; SOD = superoxide dismutase; GCLC = glutamate cysteine ligase catalytic subunit; GCLM = glutamate-cysteine ligase regulatory subunit; HO-1 = heme oxygenase-1; NQO-1 = phosphate adenine dinucleotide quinone oxidoreductase-1.
2.10. Fecal microbiota transplantation
We prepared fecal suspension according our previous protocol (Xu et al., 2021a, 2021b). Briefly, piglets’ fresh feces samples collected from EA group were immediately homogenized with O2-free saline and then passed through the sterilized gauze and then a 0.224-mm stainless cell strainer was used to remove the particles. The re-suspended fecal suspension after centrifugation at 3,500 × g for 10 min was used to treat piglets via oral administration (2 mL per piglet every other day).
2.11. Gut microbiota profiling
The total genomic DNA of fecal bacteria was extracted using the TGuide S96 Magnetic Soil/Stool DNA Kit (TIANGEN, China) according to manufacturer's instructions. The integrity of DNA was assessed by agarose gel electrophoresis. The genomic DNA was used as a template for PCR amplification. Universal primers 515F and 806R were used for PCR amplification of the V4 hypervariable regions of 16S rRNA genes (515F, 5′-GTGYCAGCMGCCGCGGTAA-3′; 806R, 5′-GGACTACNVGGGTWTCTAAT-3′). The generated DNA pool was then paired end sequenced (2 × 250) on an Novaseq 6000 platform (Illumina, San Diego, USA) at Biomarker Technologies Co, Ltd. (Beijing, China).
We analyzed sequencing raw data using the Quantitative Insights into Microbial Ecology software package (version 2021.6) (Bolyen et al., 2019). USEARCH (Edgar, 2013) (version 10.0) was employed to cluster sequences into operational taxonomic units (OTUs) with similarity over 97%. The taxonomy of each OTU representative sequence was analyzed using Ribosomal Database Project Classifier v.2.2 trained on the database Greengene_2013_5_99 and Silva (Release132) (Quast et al., 2013; DeSantis et al., 2006) with 0.6 confidence values as cutoff. The alpha diversity indices including Observed species, Chao1 value, ACE value, Shannon, and Simpson value were calculated by Mothur (v1.31.2) (Schloss et al., 2009) with the corresponding rarefaction curve drawn by software R (version 4.3.1). We performed principal co-ordinates analysis (PCoA) analysis and permutational multivariate analysis of variance (PERMANOVA, with 999 Monte Carlo permutations) based on Bray–Curtis distances using the package “vegan” in R software (version 4.3.1) (Wang et al., 2019). We identified different bacteria using linear discriminant analysis effect size analysis (Segata et al., 2011).
2.12. Gut microbial function and metabolites analysis
We employed PICRUSt to predict the function of gut microbiota based on 16S rDNA data and reference genome (Douglas et al., 2020). Then, gas chromatography was used to determine the gut microbial metabolites short chain fatty acids (SCFA) including acetic acid, propionic acid, butyric acid, valeric acid, isobutyric acid, and isovaleric acid in jejunal content and colonic content (Yan et al., 2019).
2.13. Data processing and statistics analysis
Experimental data were analyzed using Excel 2016 for one-way ANOVA and the Duncan multiple comparison test with GraphPad 8.0 software. Results were presented as mean ± SEM and the significance was presented as ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. P-values between 0.05 and 0.10 were considered as indicative of a trend.
3. Results
3.1. Dietary EA supplementation and FMT improved diarrhea, intestinal barrier function and redox imbalance in weaning piglets
To determine the effect of dietary EA supplementation on weaning stress in piglets and the role of gut microbiota in this effect, we treated weanling piglets with dietary EA supplementation and transferred their fecal microbiota to piglets in FEA group. As shown in Table 2, EA increased ADFI on d 14 (P < 0.05), ADG on d 7 (P < 0.05) and d 14 (P < 0.01) compared with the Ctrl group. FEA group increased ADG on d 14 (P < 0.05) compared with the Ctrl group.
Table 2.
Effects of ellagic acid (EA) and fecal microbiota transplantation (FMT) on the growth performance of weaned piglets.1
| Item | Ctrl group | EA group | FEA group |
|---|---|---|---|
| Day 0 to 7 | |||
| BW at d 0, kg | 6.33 ± 1.38 | 6.35 ± 0.60 | 5.63 ± 0.75 |
| BW at d 7, kg | 6.81 ± 1.38 | 6.86 ± 0.62 | 6.16 ± 0.77 |
| ADFI, g | 145.02 ± 35.15 | 168.62 ± 20.33 | 153.01 ± 28.65 |
| ADG, g | 66.69 ± 7.96b | 80.24 ± 5.90a | 76.53 ± 7.52ab |
| F:G ratio | 2.09 ± 0.11 | 1.90 ± 0.63 | 1.83 ± 0.53 |
| Day 8 to 14 | |||
| BW at d 14, kg | 7.27 ± 1.39 | 6.54 ± 0.63 | 5.86 ± 0.77 |
| ADFI, g | 176.42 ± 17.41b | 206.69 ± 28.98a | 198.28 ± 40.07ab |
| ADG, g | 70.28 ± 8.99c | 91.76 ± 8.76a | 89.53 ± 10.36b |
| F:G ratio | 2.40 ± 0.14 | 1.84 ± 0.76 | 1.91 ± 0.77 |
ADFI = average daily feed intake; ADG = average daily weight gain; F:G ratio = ratio of feed to gain; d 0 = the day of weaning; d 7 = day 7 post weaning; d 14 = day 14 post weaning.
ab Within a row, means without a common superscript differs (P < 0.05).
Data presented as mean ± SEM. Ctrl group, piglets were fed the basal diet; EA group, piglets were fed the basal diet supplemented with ellagic acid; FEA group, piglets were fed the basal diet and received FMT from EA-treated piglets.
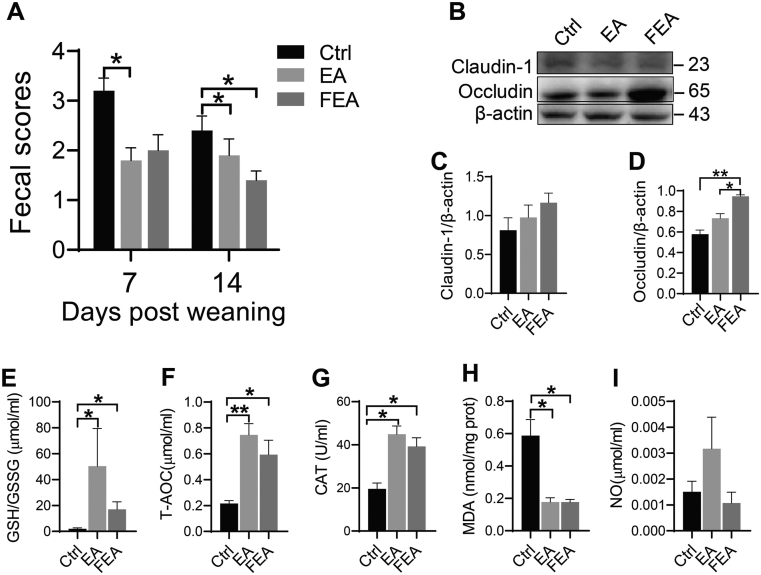
The effects of dietary EA supplementation and FMT on diarrhea, intestinal damage, and redox imbalance in weanling piglets are shown in Fig. 1. Compared with the Ctrl group, EA group decreased piglets’ fecal scores on d 7 (P < 0.05) and d 14 (P < 0.05), while FEA group decreased the scores on d 14 (P < 0.05), but not on d 7 (Fig. 1A). Compared with the Ctrl group, EA group (P < 0.05) and FEA group (P < 0.01) both increased the expression of the tight junction protein occludin, but had no effect on claudin-1 expression (Fig. 1B). In addition, EA increased antioxidant indices, including GSH/GSSG (Fig. 1C, P < 0.05), T-AOC (Fig. 1D, P < 0.01), and CAT (Fig. 1E, P < 0.05); and reduced oxidative metabolite MDA (Fig. 1F, P < 0.05) in jejunum tissue compared with the Ctrl group. Compared with the Ctrl group, FEA group had comparable effects than EA on antioxidant indices including increased GSH/GSSG, T-AOC, and CAT (Fig. 1C–E, P < 0.05), and reduced oxidative metabolite MDA (Fig. 1F, P < 0.05). Both EA and FEA groups had no significant effect on NO level (Fig. 1G, P > 0.05).
Fig. 1.
The effects of dietary ellagic acid (EA) supplementation and fecal microbiota transplantation (FMT) on diarrhea, intestinal damage, and redox imbalance in weanling piglets. (A) Fecal scores on d 7 and 14. (B) Representative bands for Western blot. (C and D) Western blot of tight junction proteins claudin-1 and occludin in jejunum tissue, respectively. (E-I) Antioxidant indices including GSH/GSSG, T-AOC, CAT, MDA, and NO in the jejunum tissue. Ctrl, the control group, where piglets were fed the basal diet; EA, the EA group, where piglets were fed the basal diet supplemented with EA; FEA, the FEA group, where piglets were fed the basal diet and received FMT from EA-treated piglets. GSH/GSSG = glutathione/glutathione (oxidized); T-AOC = total antioxidant capacity; CAT = catalase; MDA = malondialdehyde; NO = nitric oxide. Data presented as mean ± SEM and significance was presented as ∗P < 0.05 and ∗∗P < 0.01 (n = 3).
3.2. Dietary EA supplementation and FMT improved intestinal morphology and epithelial apoptosis in weanling piglets
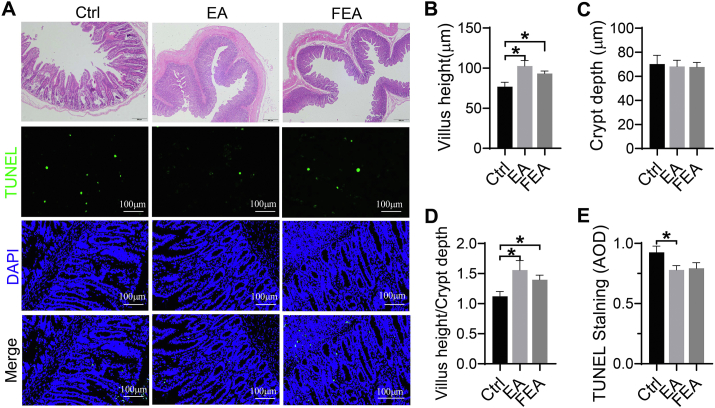
The intestinal morphology and epithelial apoptosis of weanling piglets are shown in Fig. 2. Compared with Ctrl group, EA and FEA groups ameliorated the epithelium damages of lamina propria gland and the villus integrity (Fig. 2A). EA increased villus height (P < 0.05) (Fig. 2B) and villus height-to-crypt depth ratio (P < 0.05) (Fig. 2D), but decreased the AOD of TUNEL staining (P < 0.05) (Fig. 2E) when compared with the Ctrl group. Both EA and FEA groups had no significant difference on crypt depth (Fig. 2C).
Fig. 2.
The effect of dietary ellagic acid (EA) supplementation and fecal microbiota transplantation (FMT) on intestinal morphology and epithelial apoptosis in weanling piglets. (A) H&E and TUNEL stained jejunum tissue. (B) Villus height. (C) Crypt depth. (D) Villus height-to-crypt depth ratio. (E) AOD of TUNEL staining. Scaler bar: 500 μm (H&E), 100 μm (TUNEL). Ctrl, the control group, where piglets were fed the basal diet; EA, the EA group, where piglets were fed the basal diet supplemented with EA; FEA, the FEA group, where piglets were fed the basal diet and received FMT from EA-treated piglets. AOD = average optical density, H&E = hematoxylin and eosin stain; TUNEL = terminal deoxynucle otidyl transferase dUTP nick end labeling; DAPI = 4′,6-diamidino-2-phenylindole. Data presented as mean ± SEM and significance was presented as ∗P < 0.05 (n = 7).
3.3. Dietary EA supplementation and FMT regulated the expression of genes related to inflammatory cytokines and antioxidant indicators in weanling piglets
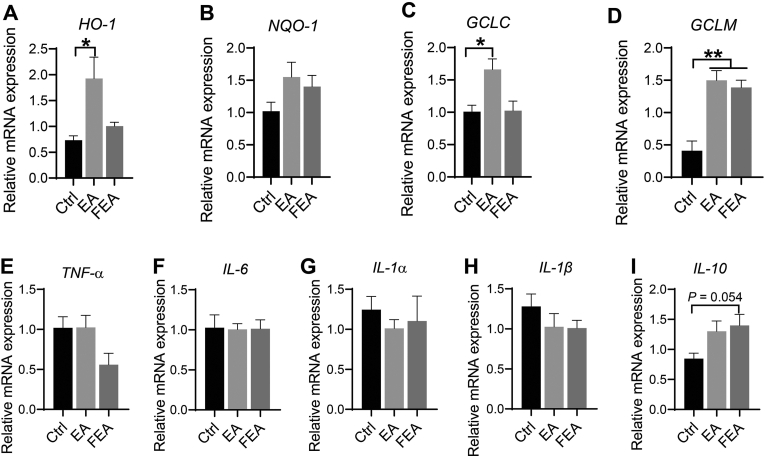
The expression levels of antioxidant indices and inflammatory factor-related genes in the jejunal tissue are shown in Fig. 3. Compared with the Ctrl group, EA group improved the antioxidant capacity of weanling piglets by increasing the mRNA levels of HO-1(P < 0.05) (Fig. 3A), GCLC (P < 0.05) (Fig. 3C), and GCLM (P < 0.01) (Fig. 3D) in jejunum tissues. FEA group increased mRNA level of GCLM (P < 0.01) (Fig. 3D). Both EA and FEA groups had no statistically significant influence on the mRNA levels of inflammatory cytokines, although FEA group tended to increase the mRNA levels of the anti-inflammatory cytokine IL-10 (Fig. 3I) compared with the Ctrl group.
Fig. 3.
The effect of dietary ellagic acid (EA) supplementation and fecal microbiota transplantation (FMT) on the gene expression of inflammatory cytokines and antioxidant factors in weanling piglets. The genes of antioxidant indices in jejunum tissues including the following: (A) HO-1, (B) NQO-1, (C) GCLC, and (D) GCLM. The genes of inflammatory cytokines in jejunum tissues including the following: (E) TNF-α, (F) IL-6, (G) IL-1α, (H) IL-1β, and (I) IL-10. Ctrl, the control group, where piglets were fed the basal diet; EA, the EA group, where piglets were fed the basal diet supplemented with EA; FEA, the FEA group, where piglets were fed the basal diet and received FMT from EA-treated piglets. HO-1 = heme oxygenase −1; NQO-1 = quinone oxidoreductase-1; GCLC = glutamate cysteine ligase catalytic subunit; GCLM = glutamate cysteine ligase regulatory subunit; TNF-α = tumor necrosis factor–α; IL-6 = Interleukin-6; IL-1β = interleukin-1β; IL-1α = interleukin-1a; and IL-10 = interleukin-10. Data presented as mean ± SEM and significance was presented as ∗P < 0.05 and ∗∗P < 0.01 (n = 7).
3.4. Dietary EA supplementation and FMT shifted gut microbial diversity in weanling piglets
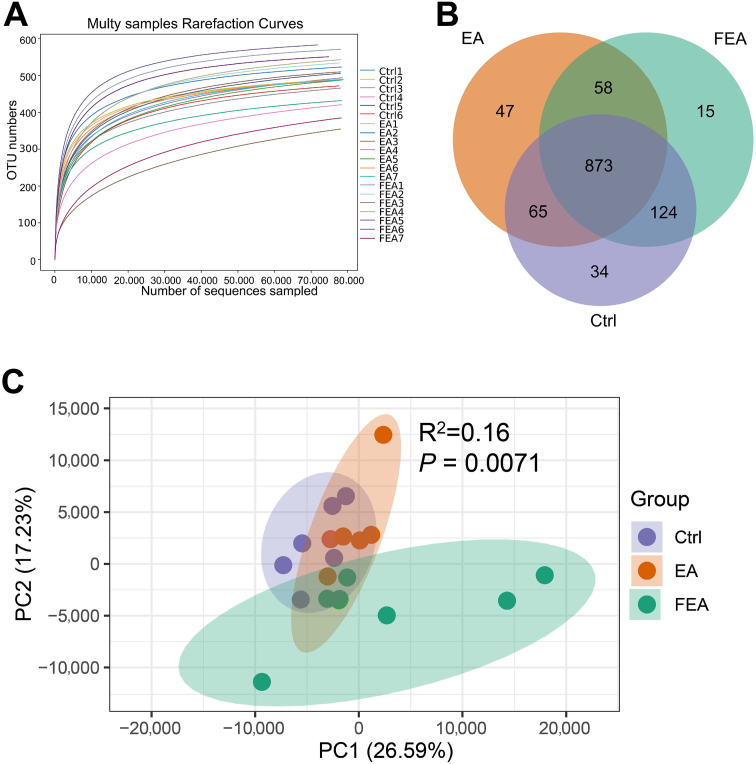
We determined shifts in the gut microbiota of piglets using 16S rDNA amplicon high-throughput sequencing. The curves in Fig. 4A tend to be flat, indicating that there were sufficient sequencing data to detect all species. The OTU distribution among the groups showed that EA group shared more OTUs with FEA group than with the Ctrl group (Fig. 4B). PCoA showed that the gut microbiota among the groups was significantly different (Fig. 4C, P = 0.0071 by PERMANOVA). Alpha diversity analysis showed that EA increased the Observed species (P < 0.05), but had no statistically significant effect on Chao1, Shannon, Simpson, and Coverage compared to the Ctrl group (Table 4). Compared with the Ctrl group, both EA and FEA groups increased ACE although the difference was not statistically significant.
Fig. 4.
The effect of dietary ellagic acid (EA) supplementation and fecal microbiota transplantation (FMT) on the gut microbiota in weanling piglets. (A) Rarefaction curve of species counts. (B) Venn diagram of OTU distribution among groups. (C) The structure shifts (beta diversity) presented by PCoA plot based on Bray–Curtis distances and assessed by PERMANOVA analysis. Ctrl, the control group, where piglets were fed the basal diet; EA, the EA group, where piglets were fed the basal diet supplemented with EA; FEA, the FEA group, where piglets were fed the basal diet and received FMT from EA-treated piglets. OTU = operational taxonomic units; PCoA = principal coordinates analysis. Data presented as mean ± SEM. (n = 7).
Table 4.
Effects of ellagic acid (EA) and fecal microbiota transplantation (FMT) on the gut microbial diversity of weanling piglets.1
| Item | Ctrl group | EA group | FEA group |
|---|---|---|---|
| Alpha diversity | |||
| Observed species | 440.67 ± 30.82ac | 485.71 ± 23.32ab | 491.71 ± 104.62a |
| ACE | 508.45 ± 31.25 | 531.50 ± 21.17 | 560.24 ± 69.15 |
| Chao1 | 521.58 ± 35.40 | 537.86 ± 21.98 | 567.64 ± 76.15 |
| Simpson | 0.96 ± 0.016 | 0.97 ± 0.012 | 0.94 ± 0.033 |
| Shannon | 5.85 ± 0.50 | 6.18 ± 0.35 | 5.57 ± 1.011 |
| Coverage | 0.99 ± 0.00010 | 0.99 ± 0.00010 | 0.99 ± 0.00020 |
abc Within a row, means without a common superscript differs (P < 0.05).
Data presented as mean ± SEM. Ctrl group, piglets were fed the basal diet; EA group, piglets were fed the basal diet supplemented with ellagic acid; FEA group, piglets were fed the basal diet and received FMT from EA-treated piglets.
3.5. Dietary EA supplementation and FMT altered gut microbial structure in weanling piglets
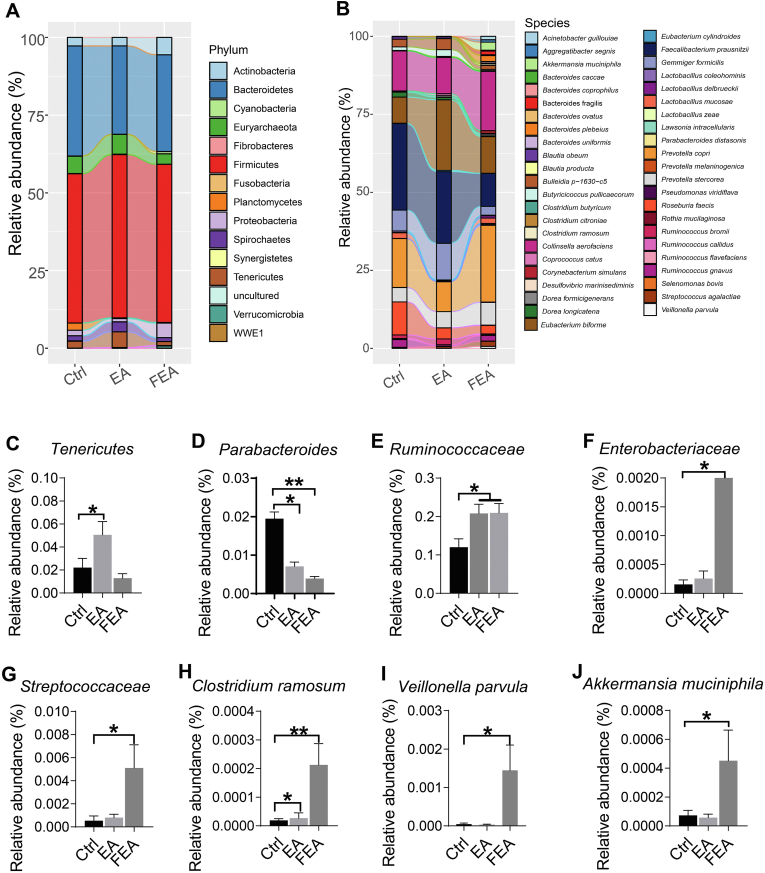
Compared with the Ctrl group, the linked bar plots of the taxon abundance illustrated that EA and FEA groups shifted the relative abundance of bacteria at different taxon levels including phylum (Fig. 5A) and species (Fig. 5B). Compared with the Ctrl group, EA group increased the relative abundance of Tenericutes (P < 0.05, Fig. 5C), Ruminococcaceae (P < 0.05, Fig. 5E), and Clostridium ramosum (P < 0.01, Fig. 5H), but decreased the relative abundances of Parabacteroides (P < 0.05, Fig. 5D); FEA group increased the relative abundances of Ruminococcaceae (P < 0.05, Fig. 5E), Enterobacteriaceae (P < 0.05, Fig. 5F), Fibrobacteres (P < 0.05, Fig. 5G), C. ramosum (P < 0.01, Fig. 5G), Veillonella parvula (P < 0.05, Fig. 5I), and Akkermansia muciniphila (P < 0.05, Fig. 5J), but decreased the relative abundance of Parabacteroides (P < 0.01, Fig. 5D).
Fig. 5.
The effect of dietary ellagic acid (EA) supplementation and fecal microbiota transplantation (FMT) on the taxon abundance of gut microbiota in weanling piglets. (A and B) The relative abundance of gut microbiota at levels of phylum and species, respectively. (C-J) The relative abundance of differential bacteria among groups. Ctrl, the control group, where piglets were fed the basal diet; EA, the EA group, where piglets were fed the basal diet supplemented with EA; FEA, the FEA group, where piglets were fed the basal diet and received FMT from EA-treated piglets. Data presented as mean ± SEM and significance was presented as *P < 0.05 and **P < 0.01 (n = 7).
3.6. Dietary EA supplementation and FMT altered the function and metabolites of the gut microbiota in weanling piglets
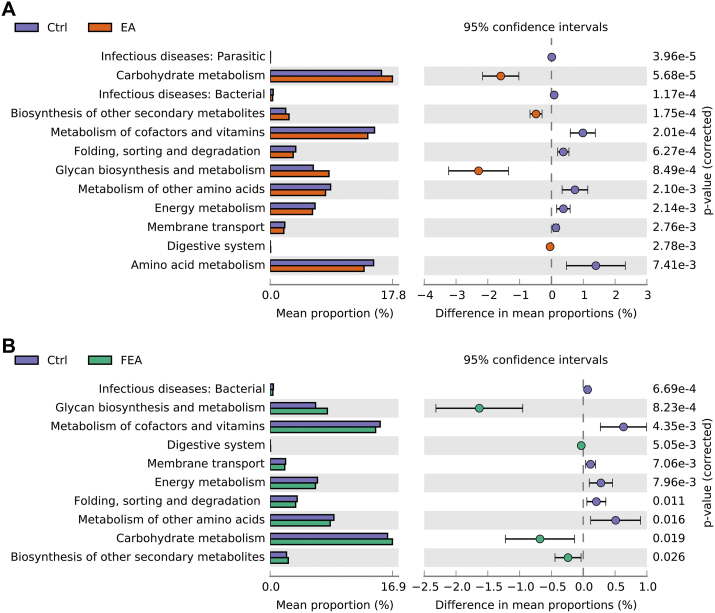
We determined the functional shifts of the gut microbiota using PICRUSt2 and further analyzed their differences with STAMP (Parks et al., 2014). Compared with the Ctrl group, EA group increased the functions of carbohydrate metabolism, biosynthesis of other secondary metabolites, glycan biosynthesis and metabolism, and digestive system, but decreased the functions of infectious parasitic diseases, infectious bacterial diseases, metabolism of cofactors and vitamins, folding, sorting and degradation, metabolism of other amino acids, energy metabolism, membrane transport, and amino acid metabolism (P < 0.01, Fig. 6A), and FEA group increased the functions of glycan biosynthesis and metabolism, digestive system, carbohydrate metabolism, and biosynthesis of other secondary metabolites, but decreased the functions of infectious bacterial diseases, metabolism of cofactors and vitamins, membrane transport, energy metabolism, folding, sorting and degradation, and metabolism of other amino acids (P < 0.05, Fig. 6B).
Fig. 6.
The effect of dietary ellagic acid (EA) supplementation and fecal microbiota transplantation (FMT) on the function of gut microbiota in weanling piglets. Different functional composition of gut microbiota: (A) Ctrl vs. EA, (B) Ctrl vs. FEA. Ctrl, the control group, where piglets were fed the basal diet; EA, the EA group, where piglets were fed the basal diet supplemented with EA; FEA, the FEA group, where piglets were fed the basal diet and received FMT from EA-treated piglets. Data presented as mean ± SEM (n = 7).
Among the shifted gut microbial functions, Carbohydrate Metabolism function refers to the gut microbiota fermenting carbohydrates in a strictly anaerobic environment to produce SCFA. Compared with the Ctrl group, EA group not only increased concentrations of acetic acid (P < 0.05), propionic acid (P < 0.01), butyric acid (P < 0.05), and total SCFA (P < 0.001) in colonic content, but also increased the acetic acid and total SCFA in jejunal content (P < 0.05), while FEA group increased the concentrations of propionic acid (P < 0.05) and total SCFA in colonic content (P < 0.01), and also increased the total SCFA in jejunal content (P < 0.05) (Table 5).
Table 5.
Short chain fatty acid (SCFA) concentrations in piglets’ colonic and jejunal content.1
| Item, μmol/g | Ctrl group | EA group | FEA group |
|---|---|---|---|
| Colonic content | |||
| Acetic acid | 72.56 ± 13.46b | 99.66 ± 19.05a | 86.35 ± 10.47ab |
| Propionic acid | 51.70 ± 14.83c | 79.32 ± 10.81a | 68.54 ± 8.36b |
| Butyric acid | 22.75 ± 8.63 | 37.55 ± 7.76 | 31.30 ± 7.11 |
| Isobutyric acid | 5.65 ± 3.52 | 6.85 ± 3.48 | 7.71 ± 4.26 |
| Isovaleric acid | 3.21 ± 2.93 | 5.18 ± 3.06 | 5.30 ± 2.40 |
| Valeric acid | 2.90 ± 1.33 | 4.85 ± 1.61 | 4.89 ± 1.82 |
| Total SCFA | 158.76 ± 18.41c | 233.40 ± 28.69a | 204.09 ± 13.82b |
| Jejunal content | |||
| Acetic acid | 16.61 ± 3.86b | 24.83 ± 6.92a | 21.65 ± 3.88ab |
| Propionic acid | 2.28 ± 1.08 | 3.51 ± 1.11 | 3.20 ± 0.91 |
| Butyric acid | 1.16 ± 0.33 | 1.77 ± 0.69 | 1.42 ± 0.23 |
| Isobutyric acid | 0.12 ± 0.04 | 0.17 ± 0.04 | 0.16 ± 0.04 |
| Isovaleric acid | 0.56 ± 0.18 | 0.65 ± 0.14 | 0.62 ± 0.13 |
| Valeric acid | 0.14 ± 0.03 | 0.17 ± 0.06 | 0.18 ± 0.08 |
| Total SCFA | 20.87 ± 3.41c | 31.08 ± 6.86a | 27.22 ± 3.40b |
abc Within a row, means without a common superscript differs (P < 0.05).
Data presented as mean ± SEM. Ctrl group, piglets were fed the basal diet; EA group, piglets were fed the basal diet supplemented with ellagic acid (EA); FEA group, piglets were fed the basal diet and received fecal microbiota transplantation from EA-treated piglets.
3.7. Dietary EA supplementation attenuated intestinal damage and oxidative stress in association with gut microbiota in weanling piglets
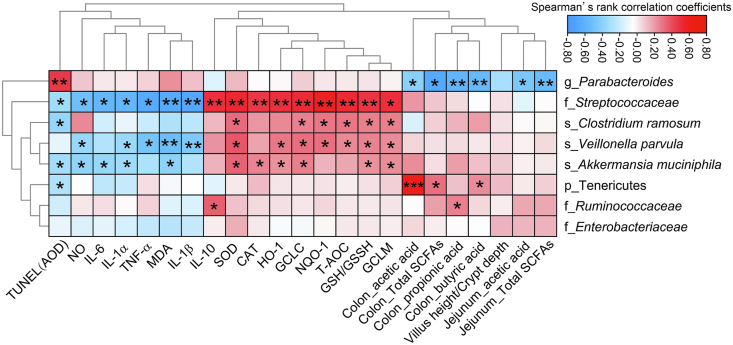
The Spearman rank correlation coefficient and significance testing (Fig. 7) showed that Parabacteroides was negatively correlated with jejunum_total SCFA (P < 0.01), jejunum_acetic acid (P < 0.05), colon_butyric acid (P < 0.01), colon_propionic acid (P < 0.01), colon_total SCFA (P < 0.05), and colon_acetic acid (P < 0.05); but was positively correlated with TUNEL(AOD) (P < 0.01). Fibrobacteres was positively correlated with the expression of antioxidant-related genes (GCLM, NQO-1, GCLC, and HO-1) (P < 0.05), antioxidant indices (GSH/GSSH, T-AOC, CAT, and SOD) (P < 0.05), and anti-inflammatory factor IL-10 (P < 0.01); but was negatively correlated with TUNEL(AOD) (P < 0.05), NO (P < 0.05), MDA (P < 0.05), and the pro-inflammatory factors (IL-6, IL-1α, TNF-α, MDA, and IL-1β) (P < 0.05). C. ramosum was positively correlated with GCLM (P < 0.05), NQO-1(P < 0.05), GCLC (P < 0.05), GSH/GSSH (P < 0.05), T-AOC (P < 0.05), and SOD (P < 0.05), but negatively correlated with TUNEL(AOD) (P < 0.05). V. parvula was positively correlated with GCLM (P < 0.05), NQO-1(P < 0.05), GCLC (P < 0.05), HO-1(P < 0.05), GSH/GSSH (P < 0.05), T-AOC (P < 0.05), and SOD (P < 0.05), but negatively correlated with IL-1β (P < 0.01), MDA (P < 0.01), TNF-α (P < 0.05), IL-1α (P < 0.05), and NO (P < 0.05). A. muciniphila was positively correlated with GCLM (P < 0.05), GCLC (P < 0.05), HO-1(P < 0.05), GSH/GSSH (P < 0.05), and SOD (P < 0.05), but negatively correlated with MDA (P < 0.05), IL-1α (P < 0.05), IL-6 (P < 0.05), NO (P < 0.05), and TUNEL(AOD) (P < 0.05). Tenericutes was positively correlated with butyric acid (P < 0.05), total SCFA (P < 0.05), and acetic acid (P < 0.001) in colonic content, but negatively correlated with TUNEL(AOD) (P < 0.05). Ruminococcaceae was positively correlated with propionic acid in colonic content (P < 0.05) and IL-10 (P < 0.05).
Fig. 7.
Correlation analysis among the identified different bacteria and examined indices. Red and blue represent positive and negative correlations, respectively. P = phylum; f = family; g = genus; s = species. Significance was presented as *P < 0.05, **P < 0.01, and ***P < 0.001 (n = 7).
4. Discussion
Previous evidences suggest that anti-oxidative agents could potentially function as antibiotic alternatives to facilitate the weaning transition of piglets by restoring a balanced gut microbiota (Gresse et al., 2017). Ellagic acid, a polyphenol, is widely present in several fruits and Chinese herbs and has antioxidant activity and regulatory effects on the gut microbiota (Cornélio Cornélio Favarin et al., 2013; Zhao et al., 2021). However, the effect of EA on alleviating weaning stress in piglets and the role of gut microbiota remain unclear. Here, we determined, for the first time, the effect of dietary EA supplementation on the attenuation of intestinal damage, oxidative stress, and gut microbial dysbiosis in weanling piglets. We found that dietary supplementation with 0.1% EA (1 g/kg diet) improved diarrhea, intestinal barrier function, redox imbalance, intestinal morphology, and intestinal epithelial cell apoptosis in weanling piglets. Early weaning transition usually results in atrophy of the small intestine, reduced nutrient and electrolyte absorption, and reduced barrier function (Gresse et al., 2017). Dietary EA supplementation not only improved intestinal barrier function by increasing the expression of tight junction proteins claudin-1 and occludin, but also relieved intestinal atrophy by improving villus height, crypt depth, villus height-to-crypt depth ratio, and epithelial apoptosis in the jejunum. These improvements in intestinal function were further supported by the increased ADG and ADFI. Consistent with our results, Chen et al. (2018) found that dietary chlorogenic acid, a polyphenol and antioxidant, improved the growth performance of weaned piglets by maintaining intestinal function. Oxidative stress in the inflamed gut lumen disturbs the microbiota in oxygen-sensitive niche, thereby triggering dysbiosis of the gut microbiota (Donaldson et al., 2016; Gresse et al., 2017). Piglet weaning is associated with severe intestinal damage and oxidative stress (Campbell et al., 2013; Lallès et al., 2007b). In this study, dietary EA supplementation relieved piglet intestinal oxidative stress, as indicated by increased antioxidant indices of GSH/GSSG, T-AOC, and CAT, as well as the reduced oxidative metabolite MDA in jejunum tissue. The Nrf2 pathway can prevent and reduce cell damage caused by oxidative stress, and plays an important role in protecting the integrity of the intestinal tract by regulating the production of pro-inflammatory cytokines and inducing the production of antioxidant enzymes (Wen et al., 2019). When exposed to oxidative stress, Nrf2 migrates to the nucleus and protects the cell by inducing the expression of various antioxidant genes such as HO-1 and NQO1(Lee et al., 2019). The results showed that dietary EA supplementation increased the mRNA expression of the HO-1 gene. Glutamate cysteine ligase (GCL) catalyzes the first rate-limiting step in the production of the cellular antioxidant GSH (Franklin et al., 2009). Dietary EA supplementation increased the mRNA expression of GCLC and GCLM, which are the catalytic and regulatory subunits of GCL, respectively (Lu, 2013). These antioxidant gene expression levels were compared well with the increased antioxidant indices in jejunum tissues.
Notably, FMT from EA-treated piglets had comparable effects on attenuating diarrhea, intestinal morphology, epithelial apoptosis, intestinal barrier function, and redox imbalance, suggesting that the gut microbiota plays a vital role in relieving weaning stress in piglets. Therefore, we determined the gut microbial shifts using 16S rDNA sequencing and found that EA group and FEA group shared more OTU numbers than with the Ctrl group, indicating that FMT from EA-treated piglets shifted the piglets’ gut microbiota to EA-treated piglets. This was further supported by the beta-diversity analysis presented by PCoA. Alpha diversity analysis showed that EA increased the number of observed species, which is consistent with the results reported by Zhao et al. (2021). Taxon analysis showed that EA increased the relative abundances of Tenericutes and C. ramosum but decreased the relative abundance of Parabacteroides. FEA group increased the relative abundance of Enterobacteriaceae, Streptococcaceae, C. ramosum, V. parvula, and A. muciniphila, but decreased the relative abundance of Parabacteroides and Ruminococcaceae. Tenericutes are a unique class of bacteria that lack a cell wall and are typically parasites or commensals of eukaryotic hosts (Skennerton et al., 2016). C. ramosum is an anaerobic, spore-forming, gram-positive bacterium that promotes serotonin (5-hydroxytryptamine, 5-HT) secretion, and thereby modulating intestinal secretion and inflammation (Liu et al., 2021; Mandić et al., 2019). In agreement well with our results, Jennings et al. (2021) found that higher intakes of berries and apples/pears which contain a large group of plant-derived polyphenolic compounds were associated with a lower abundance of Parabacteroides. Enterobacteriaceae includes both beneficial commensal microbiota and opportunistic pathogens (Philips and Blaser, 2015). The increase in Enterobacteriaceae caused by FEA had no significant correlation with the examined indices, indicating that these increased bacterial species or strains belonging to Enterobacteriaceae were not harmful ones. Fibrobacteres are SCFA-producing bacteria (Dalile et al., 2019; Den Besten et al., 2013), and we found that Fibrobacteres had a significantly positive correlation with antioxidant indices and a negative correlation with pro-inflammatory factors and the apoptosis index. Recent data suggest that V. parvula may play a protective role in the development of the immune system in early childhood (Dzidic et al., 2018). In this study, V. parvula had a significantly positive correlation with antioxidant indices and negatively correlated with pro-inflammatory factors. A. muciniphila increased in FEA group and was positively correlated with antioxidant indices and negative correlation with pro-inflammatory factors, and the apoptosis index which may be due to its ani-inflammatory effect (Kim et al., 2020). Ruminococcaceae was positively correlated with IL-10, and propionic acid in colonic content, which may be due to its ability to generate SCFA (Liu et al., 2019).
We further found that EA and FEA groups both increased the functions of Carbohydrate Metabolism, Glycan biosynthesis and metabolism, Digestive system, and Biosynthesis of other secondary metabolites. These increased functions of the gut microbiota were related to metabolism function, especially Carbohydrate Metabolism, which is responsible for the production of SCFA and has been widely proven to benefit the host (Den Besten et al., 2013). These functional shifts were consistent with the increased SCFA concentrations in the colonic and jejunal contents. Gut microbiota produced SCFA play a crucial role in maintaining intestinal homeostasis (Van der Hee and Wells, 2021). Propionic acid improves intestinal barrier function, inflammation, and oxidative stress via signal transducer and activator of the transcription 3 signaling pathway (Tong et al., 2016). In addition, Reigstad et al. (2015) found that the gut microbiota was able to promote 5-HT production in the colon due to the effect of SCFA on enterochromaffin cells (Reigstad et al., 2015). The 5-HT, a metabolite of tryptophan, regulates intestinal motility, secretion, inflammation, and epithelial development (Liu et al., 2021). In addition, activation of G-protein-coupled receptor 43 by SCFA is indispensable for the normal resolution of intestinal inflammatory responses (Maslowski et al., 2009). Therefore, the increase in SCFA by EA and FEA groups may contribute to the attenuation of intestinal damage and oxidative stress in weanling piglets. Spearman rank correlation coefficient and significance testing showed that the identified different bacteria were significantly related to the examined indices, indicating that the gut microbiota contributes to the effects of EA on attenuating intestinal damage and oxidative stress in weanling piglets.
5. Conclusions
In summary, the present study demonstrated that dietary supplementation with 0.1% EA significantly improved diarrhea, growth performance, intestinal damage, gut microbial dysbiosis, and intestinal antioxidative capacity. FMT further suggested that the gut microbiota mediated these beneficial effects of dietary EA supplementation on facilitating weaning transition in piglets. Antioxidative agents, such as natural polyphenols can be used as antibiotics alternatives to improve weaning stress in piglets by restoring a balanced gut microbiota.
Author contributions
Wenxia Qin conducted this study, participated in the animal experiments, analyzed the samples and the data, wrote and revised the manuscript. Baoyang Xu designed this study, participated in the animal experiments, analyzed the data, wrote and revised the manuscript. Yuwen Chen analyzed the samples. Wenbo Yang participated in the animal experiments, analyzed the samples. Yunzheng Xu participated in the animal experiments, analyzed the samples. Juncheng Huang participated in the animal experiments. Ting Duo participated in the animal experiments. Yihua Mao participated in the animal experiments. Guozong Zhou participated in the animal experiments. Xianghua Yan designed this study. Libao Ma designed this study, wrote and revised the manuscript.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation Regional Innovation and Development Joint Fund Project (U20A2055), and Agricultural Microbiology of Large Research Infrastructures (463119009). We thank Wuhan Huayang Animal Pharmaceutical Co., Ltd. for providing piglets in this trial.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2022.08.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
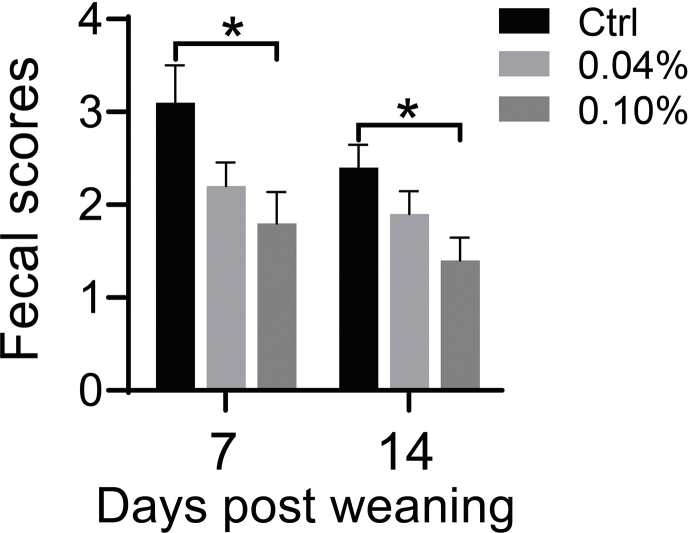
Fig. S1. The effects of dietary different levels of ellagic acid (EA) supplementation on diarrhea of weanling piglets: fecal scores on d 7 and 14 post weaning. Data presented as mean ± SEM and significance was presented as ∗P < 0.05 (n = 5).
References
- Allen H.K., Levine U.Y., Looft T., Bandrick M., Casey T.A. Treatment, promotion, commotion: antibiotic alternatives in food-producing animals. Trends Microbiol. 2013;21:114–119. doi: 10.1016/j.tim.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Allen H.K., Trachsel J., Looft T., Casey T.A. Finding alternatives to antibiotics. Ann N Y Acad Sci. 2014;1323:91–100. doi: 10.1111/nyas.12468. [DOI] [PubMed] [Google Scholar]
- Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J.M., Crenshaw J.D., Polo J. The biological stress of early weaned piglets. J Anim Sci Biotechnol. 2013;30(4):19. doi: 10.1186/2049-1891-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Li Y., Yu B., Chen D., Mao X., Zheng P., et al. Dietary chlorogenic acid improves growth performance of weaned pigs through maintaining antioxidant capacity and intestinal digestion and absorption function. J Anim Sci. 2018;96:1108–1118. doi: 10.1093/jas/skx078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornélio Favarin D., Martins Teixeira M., Lemos de Andrade E., de Freitas Alves C., Lazo Chica J.E., Artério Sorgi C., et al. Anti-inflammatory effects of ellagic acid on acute lung injury induced by acid in mice. Mediators Inflamm. 2013;2013 doi: 10.1155/2013/164202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalile B., Van Oudenhove L., Vervliet B., Verbeke K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- Den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D.-J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis T.Z., Hugenholtz P., Larsen N., Rojas M., Brodie E.L., Keller K., et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson G.P., Lee S.M., Mazmanian S.K. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol. 2016;14:20–32. doi: 10.1038/nrmicro3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S., Zhu M., Wang K., Zhao X., Hu L., Jing W., et al. Dihydromyricetin improves DSS-induced colitis in mice via modulation of fecal-bacteria-related bile acid metabolism. Pharmacol Res. 2021;171 doi: 10.1016/j.phrs.2021.105767. [DOI] [PubMed] [Google Scholar]
- Douglas G.M., Maffei V.J., Zaneveld J.R., Yurgel S.N., Brown J.R., Taylor C.M., et al. PICRUSt2 for prediction of metagenome functions. Nat Biotechnol. 2020;38:685–688. doi: 10.1038/s41587-020-0548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzidic M., Boix-Amorós A., Selma-Royo M., Mira A., Collado M.C. Gut microbiota and Mucosal Immunity in the Neonate. Med Sci (Basel) 2018;6(3):56. doi: 10.3390/medsci6030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- Franklin C.C., Backos D.S., Mohar I., White C.C., Forman H.J., Kavanagh T.J. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol Aspects Med. 2009;30:86–98. doi: 10.1016/j.mam.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S.A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresse R., Chaucheyras-Durand F., Fleury M.A., Van de Wiele T., Forano E., Blanquet-Diot S. Gut microbiota dysbiosis in Postweaning piglets: Understanding the Keys to health. Trends Microbiol. 2017;25:851–873. doi: 10.1016/j.tim.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Jennings A., Koch M., Bang C., Franke A., Lieb W., Cassidy A. Microbial diversity and abundance of Parabacteroides mediate the Associations between higher intake of Flavonoid-Rich Foods and lower blood Pressure. Hypertension. 2021;78:1016–1026. doi: 10.1161/HYPERTENSIONAHA.121.17441. [DOI] [PubMed] [Google Scholar]
- Jensen P. Observations on the maternal behaviour of free-ranging domestic pigs. Appl Anim Behav Sci. 1986;16:131–142. [Google Scholar]
- Kim Y., Hwang S.W., Kim S., Lee Y.S., Kim T.Y., Lee S.H., et al. Dietary cellulose prevents gut inflammation by modulating lipid metabolism and gut microbiota. Gut Microb. 2020;11:944–961. doi: 10.1080/19490976.2020.1730149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallès J.P., Bosi P., Smidt H., Stokes C.R. Weaning — a challenge to gut physiologists. Livest Sci. 2007;108:82–93. [Google Scholar]
- Lallès J.P., Bosi P., Smidt H., Stokes C.R. Nutritional management of gut health in pigs around weaning. Proc Nutr Soc. 2007;66:260–268. doi: 10.1017/S0029665107005484. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Jo E.H., Lee B., Noh H.M., Park S., Lee Y.M., et al. Soshiho-tang, a Traditional herbal Medicine, Alleviates Atopic Dermatitis Symptoms via regulation of inflammatory Mediators. Front Pharmacol. 2019;10:742. doi: 10.3389/fphar.2019.00742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Sun S., Wang P., Sun Y., Hu Q., Wang X. The Mechanism of secretion and metabolism of gut-derived 5-hydroxytryptamine. Int J Mol Sci. 2021;22:7931. doi: 10.3390/ijms22157931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Li E., Sun Z., Fu D., Duan G., Jiang M., et al. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci Rep. 2019;9:287. doi: 10.1038/s41598-018-36430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S.C. Glutathione synthesis. Biochim Biophys Acta. 2013;1830:3143–3153. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandić A.D., Woting A., Jaenicke T., Sander A., Sabrowski W., Rolle-Kampcyk U., et al. Clostridium ramosum regulates enterochromaffin cell development and serotonin release. Sci Rep. 2019;9:1177. doi: 10.1038/s41598-018-38018-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslowski K.M., Vieira A.T., Aylwin N., Jan K., Frederic S., Di Y., et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargeh H., Aliabadi F., Ajami M., Pazoki-Toroudi H. Role of polyphenols on gut microbiota and the Ubiquitin-Proteasome system in neurodegenerative diseases. J Agric Food Chem. 2021;69:6119–6144. doi: 10.1021/acs.jafc.1c00923. [DOI] [PubMed] [Google Scholar]
- NRC . 11th ed. National Academic Press; Washington, DC: 2012. Nutrient requirements of swine. 2012 Press. [Google Scholar]
- Parks D.H., Tyson G.W., Hugenholtz P., Beiko R.G. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30:3123–3124. doi: 10.1093/bioinformatics/btu494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips J.A., Blaser M.J. 195 - Introduction to bacteria and bacterial diseases. Bennett, Dolin, Blaser. Mandell, Douglas, and Bennett's Principles and Practice of infectious diseases. 8th ed. Elsevier; 2015. pp. 2234–2236. [Google Scholar]
- Prates J.A.M., Freire J.P.B., de Almeida A.M., Martins C., Ribeiro D.M., Osório H., et al. Influence of dietary supplementation with an amino acid Mixture on inflammatory Markers, immune Status and Serum Proteome in LPS-challenged weaned piglets. Animals. 2021;11:1143. doi: 10.3390/ani11041143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y., Yang J., Wang L., Yang X., Gao K., Zhu C., et al. Dietary resveratrol attenuation of intestinal inflammation and oxidative damage is linked to the alteration of gut microbiota and butyrate in piglets challenged with deoxynivalenol. J Anim Sci Biotechnol. 2021;12:71. doi: 10.1186/s40104-021-00596-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reigstad C.S., Salmonson C.E., Iii J.F.R., Szurszewski J.H., Linden D.R., Sonnenburg J.L., et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29:1395–1403. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss P.D., Westcott S.L., Ryabin T., Hall J.R., Hartmann M., Hollister E.B., et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S., et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skennerton C.T., Haroon M.F., Briegel A., Shi J., Jensen G.J., Tyson G.W., et al. Phylogenomic analysis of Candidatus 'Izimaplasma' species: free-living representatives from a Tenericutes clade found in methane seeps. ISME J. 2016;10:2679–2692. doi: 10.1038/ismej.2016.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke I., Pieper R., Neumann K., Zentek J., Vahjen W. The impact of high dietary zinc oxide on the development of the intestinal microbiota in weaned piglets. FEMS Microbiol Ecol. 2014;87:416–427. doi: 10.1111/1574-6941.12233. [DOI] [PubMed] [Google Scholar]
- Tong L-c, Wang Y., Wang Z-b, Liu W-y, Sun S., Li L., et al. Propionate ameliorates dextran sodium sulfate-induced colitis by improving intestinal barrier function and reducing inflammation and oxidative stress. Front Pharmacol. 2016;7:253. doi: 10.3389/fphar.2016.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Hee B., Wells J.M. Microbial regulation of host Physiology by short-chain fatty acids. Trends Microbiol. 2021;29:700–712. doi: 10.1016/j.tim.2021.02.001. [DOI] [PubMed] [Google Scholar]
- Walsh T.R., Wu Y. China bans colistin as a feed additive for animals. Lancet Infect Dis. 2016;16:1102–1103. doi: 10.1016/S1473-3099(16)30329-2. [DOI] [PubMed] [Google Scholar]
- Wang X., Tsai T., Deng F., Wei X., Chai J., Knapp J., et al. Longitudinal investigation of the swine gut microbiome from birth to market reveals stage and growth performance associated bacteria. Microbiome. 2019;7:109. doi: 10.1186/s40168-019-0721-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Zeng M., Wang Z., Qin F., Chen J., He Z. Dietary polyphenols to Combat nonalcoholic fatty liver disease via the gut–brain–liver Axis: a Review of Possible Mechanisms. J Agric Food Chem. 2021;69:3585–3600. doi: 10.1021/acs.jafc.1c00751. [DOI] [PubMed] [Google Scholar]
- Wen Z., Liu W., Li X., Chen W., Liu Z., Wen J., et al. A protective role of the NRF2-Keap1 pathway in maintaining intestinal barrier function. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/1759149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Li P., An Y., Ren J., Yan D., Cui J., et al. Phloretin ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by regulating the gut microbiota. Pharmacol Res. 2019;150 doi: 10.1016/j.phrs.2019.104489. [DOI] [PubMed] [Google Scholar]
- Wu Z., Huang S., Li T., Li N., Han D., Zhang B., et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome. 2021;9:184. doi: 10.1186/s40168-021-01115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B., Qin W., Xu Y., Yang W., Chen Y., Huang J., et al. Dietary Quercetin supplementation attenuates diarrhea and intestinal damage by regulating gut microbiota in weanling piglets. Oxid Med Cell Longev. 2021;2021 doi: 10.1155/2021/6221012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Xu B., Qin W., Yan Y., Tang Y., Zhou S., Huang J., et al. Gut microbiota contributes to the development of endometrial glands in gilts during the ovary-dependent period. J Anim Sci Biotechnol. 2021;12:57. doi: 10.1186/s40104-021-00578-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Xu B., Yin B., Xu X., Niu Y., Tang Y., et al. Modulation of gut microbial community and metabolism by dietary Glycyl-glutamine supplementation may Favor weaning transition in piglets. Front Microbiol. 2019;10:3125. doi: 10.3389/fmicb.2019.03125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdankhah S., Rudi K., Bernhoft A. Zinc and copper in animal feed - development of resistance and co-resistance to antimicrobial agents in bacteria of animal origin. Microb Ecol Health Dis. 2014;9:25. doi: 10.3402/mehd.v25.25862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi G.F., Carroll J.A., Allee G.L., Gaines A.M., Kendall D.C., Usry J.L., et al. Effect of glutamine and spray-dried plasma on growth performance, small intestinal morphology, and immune responses of Escherichia coli K88+-challenged weaned pigs. J Anim Sci. 2005;83:634–643. doi: 10.2527/2005.833634x. [DOI] [PubMed] [Google Scholar]
- Zhao L., Mehmood A., Soliman M.M., Iftikhar A., Iftikhar M., Aboelenin S.M., et al. Protective effects of ellagic acid against alcoholic liver disease in mice. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.744520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Jiang Q. Roles of the polyphenol–gut microbiota Interaction in alleviating colitis and preventing colitis-associated Colorectal Cancer. Adv Nutr. 2021 Mar 31;12(2):546–565. doi: 10.1093/advances/nmaa104. [DOI] [PMC free article] [PubMed] [Google Scholar]