Abstract
Nonalcoholic fatty liver disease (NAFLD) affects about a third of the world’s adult population and is a major public health concern. NAFLD is defined by the presence of hepatic steatosis and the absence of other causes of liver disease. As NAFLD is closely associated with the presence of the metabolic syndrome, several experts have called for a change in nomenclature from NAFLD to metabolic-associated fatty liver disease (MAFLD) to better reflect the underlying pathophysiology of NAFLD as a metabolically driven disease and shift to a “positive” diagnostic criteria rather than one of exclusion. Recent studies have suggested that the global prevalence of MAFLD is higher than that of NAFLD, and patients with MAFLD have more metabolic comorbidities compared to those with NAFLD. Emerging data also suggest that all-cause and cardiovascular mortality may be higher in MAFLD compared with NAFLD. In this synopsis, we discuss differences in clinical features, prevalence and clinical outcomes between NAFLD and MAFLD. In addition, we highlight the advantages and disadvantages of a name change from NAFLD to MAFLD from the perspective of the scientific community, care providers and patients.
Keywords: Nonalcoholic fatty liver disease; Nonalcoholic steatohepatitis; Liver, Nonalcoholic fatty
INTRODUCTION
The concept of nonalcoholic fatty liver disease (NAFLD) was first introduced in the early 1980s when histopathological features similar to alcohol-associated liver disease were observed in the absence of alcohol consumption [1,2], Currently, NAFLD affects 29–33% of the global population [3-5] and has emerged as a major concern for public health. The American Association for the Study of Liver Diseases (AASLD) defines NAFLD based on the presence of hepatic steatosis on imaging or biopsy and requires the exclusion of other causes of chronic liver disease [6]. NAFLD is classified into two categories: nonalcoholic fatty liver (NAFL), the benign, non-progressive form of NAFLD, and nonalcoholic steatohepatitis (NASH), the inflammatory form which may progress to cirrhosis and hepatocellular carcinoma (HCC) [6-9].
NAFLD is strongly associated with the presence of the metabolic syndrome and the rise in NAFLD closely mirrors the obesity epidemic [10,11]. Modelling studies have estimated that by 2030, 10% of the global population is projected to have diabetes [12], and nearly 50% of the USA population is projected to be obese [13]. While NAFLD affects a substantial proportion of the population, there is currently no Food and Drug Administration (FDA)-approved treatment for NAFLD, and liver transplantation is often the only treatment for patients with decompensated NASH cirrhosis [14,15]. Additionally, the presence of NAFLD results in a significantly higher risk of extrahepatic end organ damage, along with associated psychological stress [16]. Unfortunately, despite the severe morbidity and mortality burden from NAFLD, the global awareness of NAFLD remains low, particularly amongst non-hepatologists and primary care physicians who are at the frontlines managing metabolic diseases [17]. A recent international survey reported that there is a lack of emphasis on NAFLD in national health agendas in the majority of countries [18], and one third of countries scored zero on the preparedness index [19].
Meanwhile, substantial progress has been made in understanding the underlying disease mechanism of NAFLD [20]. In turn, this has lead to a call from several experts for a change in nomenclature from NAFLD to metabolic associated fatty liver disease (MAFLD), to better reflect the underlying pathophysiology of NAFLD as a metabolically driven disease [21,22]. In addition, these experts cited the absence of defined clinical criteria for a “positive” diagnosis of this disease in the traditional definition of fatty liver. Two position papers sought to integrate current understanding of patient heterogeneity captured under the acronym NAFLD and provide suggestions on terminology that can better reflect the pathogenesis of the disease [21,22]. The experts in these two papers believe that the name MAFLD more accurately describes the disease as a metabolic disorder and shifts it from a disease of exclusion to one of inclusion [21,22]. In this review, we highlight the differences in clinical features, prevalence and outcomes between NAFLD and MAFLD including the prevalence and impact of heaptic steatosis on the natural history of patients with other concurrent liver disease such as hepatitis B. In addition, we discuss the benefits, disadvantages and implications of a change in name from NAFLD to MAFLD.
DEFINITION OF NAFLD VERSUS MAFLD
An expert panel defined MAFLD as evidence of hepatic steatosis with obesity, type 2 diabetes, or ≥2 factors associated with evidence of metabolic dysfunction (Table 1) [21-23]. Importantly, the exclusion of alternative causes of chronic liver disease, such as alcohol or viral hepatitis, are no longer required to diagnose MAFLD.
Table 1.
Definitions of NAFLD and MAFLD
| Statement | Year | Definition | |
|---|---|---|---|
| NAFLD | |||
| AASLD [6] | 2018 | Evidence of hepatic steatosis on imaging or histology and a lack of secondary causes of accumulation in hepatic fat and significant alcohol consumption (>21 or 14 standard drinks per week in men and women respectively over a 2-year period). | |
| Asia Pacific Working Party [76,77] | 2017 | NAFLD is attributed to over-nutrition in the absence of other aetiology of chronic liver disease. There must be no more than one standard drink per day (70 g/week) for women or two standard drinks per day (140 g/week). | |
| EASL [78] | 2016 | Characterised by presence of steatosis in >5% of hepatocytes according to histology, proton density fat fraction or quantitative fat/water selective magnetic resonance imaging and absence of daily alcohol consumption not exceeding 30 g for men and 20 g for women. | |
| MAFLD [21-23] | 2021 | Hepatic steatosis in the presence of obesity (BMI >25 kg/m2 and >23 kg/m2 in Caucasian and Asian), type 2 diabetes mellitus or ≥2 of the following conditions: increase in waist circumference; elevated high-sensitive serum C-reactive protein level; prediabetes; elevated blood pressure; decreased HDL-cholesterol levels; increased triglycerides levels; and homeostasis model assessment (HOMA)-insulin resistance score ≥2.5. | |
NAFLD, nonalcoholic fatty liver disease; MAFLD, metabolic associated fatty liver disease; AASLD, American Association for the Study of Liver Diseases; EASL, European Association for the Study of the Liver; BMI, body mass index; HDL, high-density lipoprotein.
DIFFERENCES IN CLINICAL FEATURES BETWEEN NAFLD AND MAFLD
Risk factors
Recent data demonstrate significant differences in clinical characteristics between NAFLD and MAFLD [4]. In a recent systematic review and meta-analysis, NAFLD-MAFLD patients were more metabolically unhealthy compared to NAFLD individuals [4]. MAFLD patients had significantly higher odds of having diabetes, chronic kidney disease (CKD) and/or hypertension as compared to NAFLD patients [4]. A recent USA study used data from the Third National Health and Nutrition Examination Survey and categorized patients into three groups: non-MAFLD NAFLD, NAFLD-MAFLD, and non-NAFLD MAFLD [24]. Patients in the NAFLD-MAFLD and non-NAFLD MAFLD groups were older and had more metabolic risk factors than the non-MAFLD NAFLD group. In addition, the non-NAFLD MAFLD group were more likely to have hypertension, elevated aminotransaminases and advanced fibrosis when compared with the NAFLD-MAFLD and non-MAFLD NAFLD groups.
Prevalence
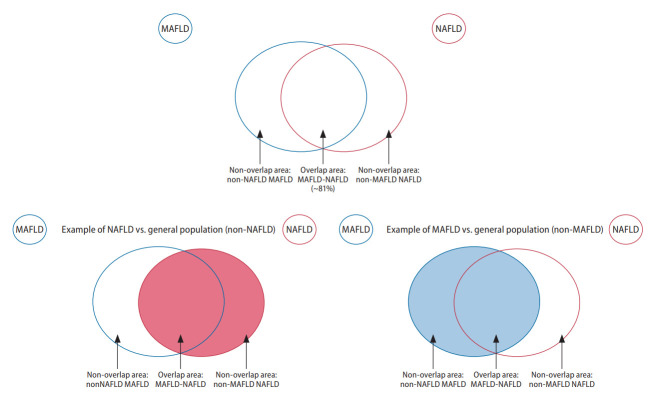
A recent meta-analysis estimated that the global prevalence of MAFLD and NAFLD to be 39% and 33%, respectively [4]. Nearly 30% to 40% of patients with hepatitis B have concurrent hepatic steatosis [25,26], and these patients were previously excluded from the definition of NAFLD. Under the new diagnostic criteria for MAFLD (Table 1), those with hepatic steatosis, concomitant metabolic dysfunction, and additional cause(s) of chronic liver disease may be included in the new definition. Only 81% of patients met criteria for both MAFLD and NAFLD, with the difference contributed by the exclusion of a substantial proportion of lean and non-obese NAFLD from MAFLD and exclusion of patients with other concomitant liver disease from NAFLD. Lean NAFLD presents a distinct subtype that accounts for 5.1% of the global population and are known to have significantly less metabolic comorbidities [27].
Outcomes
Non-NAFLD MAFLD was found to have a higher risk of all-cause mortality (hazard ratio [HR], 2.40; 95% confidence interval [CI], 1.2–4.6; P=0.01) after adjusting for age, sex, race/ethnicity, smoking, viral hepatitis, FIB-4 and weight compared to non-MAFLD NAFLD (Figs. 1, 2). Cardiovascular disease-related mortality (HR, 6.70; 95% CI, 0.9–47.1; P=0.06) was also larger in non-NAFLD MAFLD compared to non-MAFLD NAFLD after adjusting for demographic factors and provides evidence that the presence of MAFLD may be a better predictor of adverse events in the presence of hepatic steatosis [24]. The increased risk of overall mortality in the presence of MAFLD as compared to non-MAFLD was confirmed by a latter study also using the same database [28]. Interestingly, cardiovascular and cancer-related mortality were not significantly different between MAFLD and NAFLD, suggesting that cause(s) other than cardiovascular or cancer such as liver related cause may be responsible to the higher overall mortality with MAFLD [28,29]. Using data from the National Health Insurance Service in South Korea, the presence of MAFLD (vs. non-MAFLD) was associated with a significant increase in cardiovascular mortality (HR, 1.46; 95% CI, 1.41–1.52) but not the presence of NAFLD compared to non-NAFLD (HR, 1.12; 95% CI, 0.96–1.30) [30]. The risk of developing systemic end organ damage including cardiovascular disease, stroke and CKD was marginally higher in MAFLD compared to NAFLD (Table 2) after adjusting for confounders [31,32].
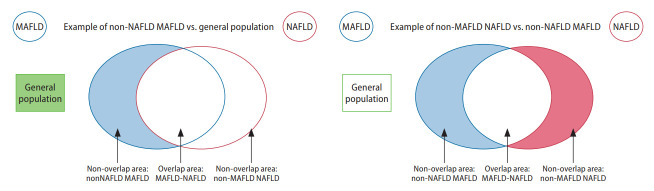
Figure 1.
Graphical illustration of groups included in the analysis of overlapping conditions. MAFLD, metabolic associated fatty liver disease; NAFLD, nonalcoholic fatty liver disease.
Figure 2.
Graphical illustration of groups included in the analysis of non-overlapping conditions. MAFLD, metabolic associated fatty liver disease; NAFLD, nonalcoholic fatty liver disease.
Table 2.
Clinical outcomes of MAFLD versus NAFLD
| Study | Database | Diagnosis | Sample size | MAFLD | NAFLD | Factors included in regression adjustment | Outcome |
|---|---|---|---|---|---|---|---|
| Huang et al. [29] | NHANES III | Ultrasound | 12,480 | 3,909 | 3,779 | Age, sex and race-ethnicity, FIB-4 score, NFS score, CRP and ALP | Overall mortality: MAFLD vs. non-MAFLD, HR 1.21 (1.09–1.33); NAFLD vs. non-NAFLD, HR 0.99 (0.81–1.20) |
| Cardiovascular mortality: MAFLD vs. non-MAFLD, HR 1.10 (0.90–1.34); NAFLD vs. non-NAFLD, HR 0.99 (0.81–1.21) | |||||||
| Neoplasm mortality: MAFLD vs. non-MAFLD, HR 1.12 (0.91–1.39); NAFLD vs. non-NAFLD, HR 0.98 (0.79–1.22) | |||||||
| Diabetes related mortality: MAFLD vs. non-MAFLD, HR 4.40 (2.49–7.76); NAFLD vs. non-NAFLD, HR 2.72 (1.59–4.63) | |||||||
| Kim et al. [28] | NHANES III | Ultrasound | 7,761 | 2,256 | 2,438 | Age, sex, race/ethnicity, education, marital status, smoking status, alanine aminotransferase, and sedentary lifestyle, body mass index, diabetes, hypertension, fasting triglycerides, high-density lipoprotein cholesterol, waist circumference and CRP | Overall mortality: MAFLD vs. non-MAFLD, HR 1.17 (1.04–1.32); NAFLD vs. non-NAFLD, HR 1.05 (0.95–1.17) |
| Cardiovascular mortality: MAFLD vs. non-MAFLD, HR 0.95 (0.75–1.21); NAFLD vs. non-NAFLD, HR 0.92 (0.71–1.17) | |||||||
| Neoplasm mortality: MAFLD vs. non-MAFLD, HR 1.15 (0.82–1.62); NAFLD vs. non-NAFLD, HR 1.02 (0.75–1.39) | |||||||
| Nguyen et al. [24] | NHAMES III | Ultrasound | 2,240 NAFLDMAFLD; 254 non-MAFLD NAFLD; 503 non-NAFLD MAFLD | Age, sex, race/ethnicity, smoking, weight categories, diabetes, hypertension, and FIB-4 categories | All-cause mortality: NAFLD-MAFLD vs. non-MAFLD NAFLD, HR 1.5 (0.8–2.8); non-NAFLD MAFLD vs. non-MAFLD NAFLD, HR 2.4 (1.2–4.6) | ||
| Cardiovascular mortality: NAFLD-MAFLD vs. non-MAFLD NAFLD, HR 3.4 (0.5–22.3); non-NAFLD MAFLD vs. non-MAFLD NAFLD, HR 6.7 (0.9–47.1) | |||||||
| Neoplasm mortality: NAFLD-MAFLD vs. non-MAFLD NAFLD, HR 1.3 (0.3–5.2); non-NAFLD MAFLD vs. non-MAFLD NAFLD, HR 2.7 (0.7–10.5) | |||||||
| Lee et al. [30] | NHIS Korea | Fatty Liver Index | 9,584,399 | 3,573,644 | 2,680,217 | Age, sex, household income quartile, residential area, Carlson Comorbidity Index, tobacco use, exercise frequency, and estimated glomerular filtration rate | Cardiovascular mortality: MAFLD vs. non-MAFLD, HR 1.46 (1.41–1.52); NAFLD vs. non-NAFLD, HR 1.12 (0.96–1.30) |
| Cardiovascular disease: MAFLD vs. non-MAFLD, HR 1.52 (1.51–1.54); NAFLD vs. non-NAFLD, HR 1.41 (1.40–1.43) | |||||||
| Myocardial infarction: MAFLD vs. non-MAFLD, HR 1.21 (1.18–1.25); NAFLD vs. non-NAFLD, HR 1.14 (1.03–1.26) | |||||||
| Ischemic stroke: MAFLD vs. non-MAFLD, HR 1.51 (1.48–1.54); NAFLD vs. non-NAFLD, HR 1.03 (0.96–1.12) | |||||||
| Heart failure: MAFLD vs. non-MAFLD, HR 1.67 (1.58–1.76); NAFLD vs. non-NAFLD, HR 0.96 (0.74–1.25) | |||||||
| Liang et al. [31] | Shanghai Nicheng Cohort Study | Ultrasound | 6,873 | 2,837 | 2,452 | Sex, age, educational background, smoking status, and leisure-time exercise at baseline | Chronic kidney disease (stage ≥3): MAFLD vs. non-MAFLD, RR 1.64 (1.39–1.94); NAFLD vs. non-NAFLD, RR 1.70 (1.43–2.01) |
| Cardiovascular disease: MAFLD vs. non-MAFLD, HR 1.44 (1.15–1.81); NAFLD vs. non-NAFLD, HR 1.48 (1.17–1.88) | |||||||
| Yoneda et al. [32] | Japan Medical Data Center | Fatty Liver Index | 1,542,688 | 237,242 | 142,158 | Age, sex, smoking habit, LDL, and statin use | Stroke: MAFLD vs. non-MAFLD, HR 1.25 (0.98–1.59); NAFLD vs. non-NAFLD, HR 0.96 (0.63–1.48) |
| Coronary artery disease: MAFLD vs. non-MAFLD, HR 1.98 (1.86–2.10); NAFLD vs. non-NAFLD, HR 1.01 (0.91–1.13) | |||||||
| Cardiovascular disease: MAFLD vs. non-MAFLD, HR 1.89 (1.78–2.01); NAFLD vs. non-NAFLD, HR 1.02 (0.92–0.14) |
MAFLD, metabolic associated fatty liver disease; NAFLD, nonalcoholic fatty liver disease; NHANES III, Third National Health and Nutrition Examination Survey; FIB-4, fibrosis-4; NFS, NAFLD fibrosis score; CRP, C-reactive protein; ALP, alkaline phosphatase; HR, hazard ratio; NHIS, National Health Insurance Service; RR, risk ratio; LDL, low-density lipoprotein.
As the diagnosis of MAFLD does not require the exclusion of another concurrent liver disease such as chronic hepatitis B, we noted here the prevalence, characteristics, and outcome of patients with fatty liver and chronic hepatitis B, a disease that affects about 290 million people worldwide [25,33,34]. A recent meta-analysis inclusive of 54 studies (28,648 patients) estimated that about one-third of patients with chronic hepatitis B (CHB) have concurrent hepatic steatosis and that the presence of hepatic steatosis was not significantly associated with significant fibrosis (odds ratio [OR], 0.87; 95% CI, 0.54–1.30; 20 studies; 6,232 patients). More recent studies have specifically examined the association between MAFLD and liver histopathology in patients with concomitant viral hepatitis. One retrospective analysis of 773 patients with biopsy-confirmed MAFLD found a higher proportion of patients with fibrosis stage 2–4 among MAFLD patients with concurrent viral hepatitis compared with patients with only MAFLD, but this study only included 40 patients with viral hepatitis which was also mixture of patients with hepatitis B virus (HBV) and patients with hepatitis C virus (HCV) infection [35]. Another recent biopsy study included a much larger cohort of patients with concomitant HBV infection (359 of the total cohort of 417; 86%) found that HBV infection was independently associated with higher grades of inflammation and fibrosis despite having an inverse association with the severity of hepatic steatosis [36]. However, the results of these two biopsy studies should be interpreted with caution as patients undergoing liver biopsy as part of their routine care are often highly selected, so the results may not be generalizable to the general population of patients with MAFLD and HBV infection. Additionally, the sample size of patients in one comparative group for both of these studies was very small (only 40 patients with MAFLD and viral hepatitis in one study and only 58 patients with MAFLD alone in the other). Regarding concurrent HCV infection and MAFLD, one study found that 43% (n=321) of 744 patients with chronic HCV infection had hepatitc steatosis on liver biopsy [37]. The study also found that concurrent MAFLD (vs. HCV infection without MAFLD) was independently associated with fibrosis stage 2–4.
In regards to long-term clinical outcomes, the presence of MAFLD-HBV was found to be associated with a higher risk of overall mortality, HCC and decompensation compared to non-MAFLD HBV [38]. HCC patients affected by both HBV and MAFLD undergoing curative resection were more likely to have recurrence compared to HCC patients with HBV infection without MAFLD [39]. However, while some studies found that patients with HBV and hepatic steatosis have worse outcomes [40,41], others have found that the presence of steatosis in HBV was asscociated with lower risk of cirrhosis, hepatocellular carcinoma, and higher chance of hepatitis B surface antigen seroclearance [42-47]. Thus, further studies are needed to elucidate the relationship between hepatic steatosis, metabolic derangement, and long-term clinical complications [42,48].
ADVANTAGES OF A CHANGE IN NAME FROM NAFLD TO MAFLD
From the perspective of the scientific community
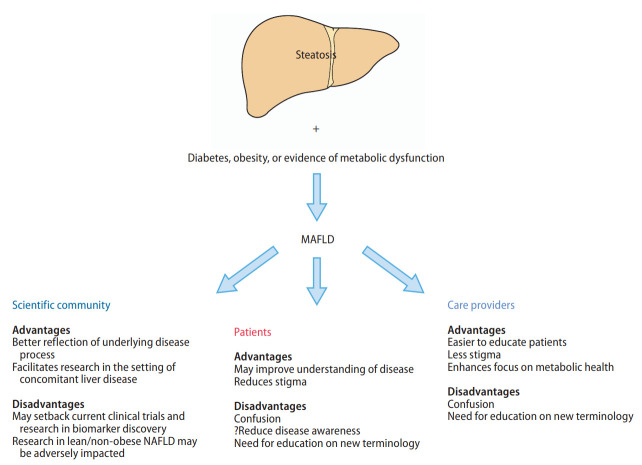
The advantages and disadvantages of a change in nomenclature are summarized in Figure 3. Significant advancements of clinical science have offered insights into the mechanisms between NAFLD, diabetes and obesity [49]. Though metabolic dysfunction is the key driver of hepatic steatosis, the current definition of NAFLD does not account for factors that are major predictors of metabolic dysfunction, such as obesity and diabetes [50]. The heterogeneity within NAFLD with respect to its primary metabolic drivers represent an important impediment to the discovery of efficacious therapies. MAFLD is a better reflection that metabolic dysregulation is the mechanism underlying the disease [51].
Figure 3.
Advantages and disadvantages of a change in name from NAFLD to MAFLD. MAFLD, metabolic associated fatty liver disease; NAFLD, nonalcoholic fatty liver disease.
Another major advantage of the change in nomenclature to MAFLD is the removal of the need to exclude other chronic liver diseases when studying patients with hepatic steatosis, such as alcohol and viral hepatitis, which would need to be excluded before a diagnosis of NAFLD can be made. For example, as one-third of the 290 million world HBV population may have hepatic steatosis [25], almost 100 million patients with concomitant CHB and hepatic steatosis would not be included under the definition of NAFLD but would be included under the umbrella term of MAFLD. Therefore, switching of nomenclature to MAFLD may help promote research and collaboration in conditions with concomitant hepatic steatosis and other causes of liver disease, such as viral hepatitis or alcohol-associated liver disease [52]. Additionally, the threshold for exclusion of significant alcohol use as required in the definition of NAFLD is often not clear. The current definition of “safe” alcohol consumption is generally defined as an intake not amounting to 30 g/day in men or 20 g/day in women, but this threshold has been hotly contested, especially in the setting of NAFLD [24,53]. A systematic review by the Global Burden of Disease confirmed that there was a lack of consensus as to what constitutes a “safe” limit in alcohol consumption [54]. Additionally, the measure of alcohol use at both patient- and population-level is often compromised by substantial recall bias despite formal quantitative alcohol consumption questionnaires as developed by the National Institute on Alcohol Abuse and Alcoholism [55,56]. As a result, a shift to use the MAFLD nomenclature may help reduce the heterogeneity and confusion for the research communities as well as clinicians caring for patients with hepatic steatosis associated with both metabolic disease and significant alcohol consumption.
Finally, since the disease burden of fatty liver disease is vast, increased engagement among the care provider community beyond liver specialists is needed, and the nomenclature that directly indicates “metabolic” derangement can help engage care providers in other disciplines such as cardiology and endocrinology in the screening, diagnosis, and management of patients with fatty liver and metabolic disease, namely MAFLD. A recent meta-analysis of over 12 million people found a significantly higher prevalence of a wide range of systemic complications in individuals with MAFLD compared to those without MAFLD, including cardiovascular disease, extrahepatic maglinancy and CKD [57]. This emphasizes the importance of multidisciplinary engagement in the management of patients with MAFLD.
From the perspective of patients and providers
NAFLD is often underdiagnosed and consequently is associated with presentation with more advanced disease and increased mortality [58]. In fact, a USA population-based study found that only 5% of people with NAFLD are aware of having a liver disease [59]. The use of the name NAFLD may contribute to poor disease awareness and understanding among patients with the disease, as patients often want to know what their disease is, not what it is not [60]. A change of nomenclature to MAFLD can remove the burden of an exhaustive work up to rule out all other causes of liver disease and having the word “metabolic” in the new nomenclature may make the disease mechanism more intuitive to both patients and care providers alike, and thus facilitating better disease understanding and management. In certain regions such as the Middle East and North Africa, the association of the name alcohol with NAFLD may also result in stigma and confusion [60], which may lead to delayed diagnosis and a higher proportion of patients being diagnosed with advanced liver disease and decompensation.
DISADVANTAGES OF A CHANGE IN NAME FROM NAFLD TO MAFLD
From the perspective of the scientific community
The change in name from NAFLD to MAFLD has been endorsed by the Chinese Society of Hepatology [61], Arabic Association for the Study of Diabetes and Metabolism [62], the Latin American Association for the Study of the Liver [63] and the Asia Pacific Association for the Study of the Liver [23]. A recent letter comprising over 1,000 signatories representing various professional bodies and physicians including hepatologists, endocrinologists, primary care physicians, nephrologists and cardiologists endorsed the change of definition to MAFLD [64]. However, to date, neither AASLD nor the European Association for the Study of the Liver have endorsed the change in nomenclature [65].
Several experts in the field have expressed concerns that a premature change in definition from one suboptimal name to another without a comprehensive assessment and consensus from all stakeholders may result in greater challenges to biomarker discovery and drug development [66]. While there are currently no FDA approved treatments for NASH, several therapies such as lanifibranor [67], semaglutide [67], and obeticholic acid [68] have demonstrated encouraging results. A sudden change in nomenclature may have a major impact the inclusion criteria for many ongoing clinical trials and may inadvertently result in delay to the approval of efficacious therapies for NAFLD. A change in name to MAFLD may also set back current progress in biomarker development, where studies have been mostly performed among patients with hepatic steatosis, without significant alcohol consumption, viral hepatitis, autoimmune liver disease, etc [69]. An example would be the discovery and development of the NIS4 which was created specifically for NASH [70]. There is an increasing recognition in the field that non-invasive tests may be appropriate endpoints for clinical trials in NAFLD; therefore, a change in disease definition may substantially set back the progress made in recent years [66],
Another implication of the change of name to MAFLD may be a reduced focus on lean or non-obese NAFLD. A recent meta-analysis estimated that the non-obese NAFLD affects 12.1% of the global population and that 40% of the global NAFLD population are non-obese [27]. In addition, among people with non-obese NAFLD, 27% have diabetes compared to only 2.4% among the general population [27]. Lean people with NAFLD may also be at higher risk of disease progression [71,72], and 6% of non-obese persons with NAFLD had metabolic disease, a proportion that is similar to that of obese people with NAFLD (61%) [73]. Further studies characterizing non-obese MAFLD are required as prior studies have suggested that non-obese NAFLD people may have higher mortality than obese people with NAFLD [71-75]. While MAFLD provides a greater focus on extrahepatic comorbidities associated with fatty liver, it potentially might result in an overemphasis on the systemic comorbidities that may result in a lack of focus on the liver itself, even though the higher risk of complications and disease could well be attributed to the presence of systemic dysregulation, rather than from the liver [57].
From the perspective of patients and care providers
In recent years, the term NAFLD has started to gain awareness among patients, primary care physicians and non-hepatology specialists [66]. Many patients with NAFLD are diagnosed by primary care physicians and non-hepatology specialists such as endocrinologists and cardiologists. A change in nomenclature from NAFLD to MAFLD may result in confusion and potentially result in even poorer disease awareness, potentially setting back the progress made in recent years.
CONCLUSION
Prior to any change in the nomenclature of the disease, it is vital that all stakeholders, including international liver societies, hepatologists, scientists, patient advocacy organizations, the bio-pharmaceutical industry, regulatory agencies, and policy makers meet and come to a consensus. Ultimately, any change in name needs to have clear advantages to patients that outweigh the potential disadvantages and consensus discussion needs to include mechanisms to address such disadvantages, such as setting back current progress in biomarker discovery and drug development for NAFLD. Any new definition would require intensive re-education of patients and care providers, particularly primary care physicians and non-hepatologist specialists. Finally, a greater emphasis must be placed on multidisciplinary and preventive care in light of the high prevalence of systemic metabolic diseases among people with metabolic-associated fatty liver disease.
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- CHB
chronic hepatitis B
- CI
confidence interval
- CKD
chronic kidney disease
- FDA
Food and Drug Administration
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- MAFLD
metabolic associated fatty liver disease
- NAFL
nonalcoholic fatty liver
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NHIS
National Health Insurance Service
- OR
odds ratio
Footnotes
Authors’ contributions
Study design: DQY, JKH, MHN; Data interpretation and review/revision of the manuscript: All authors; Study concept and study supervision: MHN; All authors approved the final draft of the manuscript as well as the authorship list.
Conflicts of Interest
Mindie H. Nguyen: Research support: Pfizer, Enanta, Gilead, Glycotest, Vir, B.K. Kee Foundation, National Cancer Institute. Advisory board/consulting: Janssen, Spring Bank, Gilead, Novartis, Bayer, Eisai, Eli Lilly, Exact Sciences, Laboratory of Advanced Medicine, Helio Health, Intercept.
Daniel Q. Huang: Research support: Exxon Mobil-NUS Research Fellowship for Clinicians, NMRC Research Training Fellowship. Advisory board/consulting: Eisai.
Other authors have no disclosures.
REFERENCES
- 1.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 2.Fleming KA, Morton JA, Barbatis C, Burns J, Canning S, McGee JO. Mallory bodies in alcoholic and non-alcoholic liver disease contain a common antigenic determinant. Gut. 1981;22:341–344. doi: 10.1136/gut.22.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, et al. 2019 Global NAFLD prevalence: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021 Dec 7; doi: 10.1016/j.cgh.2021.12.002. doi: [DOI] [PubMed] [Google Scholar]
- 4.Lim GEH, Tang A, Ng CH, Chin YH, Lim WH, Tan DJH, et al. An observational data meta-analysis on the differences in prevalence and risk factors between MAFLD vs NAFLD. Clin Gastroenterol Hepatol. 2021 Dec 4; doi: 10.1016/j.cgh.2021.11.038. doi: [DOI] [PubMed] [Google Scholar]
- 5.Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4:389–398. doi: 10.1016/S2468-1253(19)30039-1. [DOI] [PubMed] [Google Scholar]
- 6.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 7.Muthiah MD, Cheng Han N, Sanyal AJ. A clinical overview of non-alcoholic fatty liver disease: a guide to diagnosis, the clinical features, and complications-what the non-specialist needs to know. Diabetes Obes Metab. 2022;24 Suppl 2:3–14. doi: 10.1111/dom.14521. [DOI] [PubMed] [Google Scholar]
- 8.Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223–238. doi: 10.1038/s41575-020-00381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang DQ, Fowler KJ, Liau J, Cunha GM, Louie AL, An JY, et al. Comparative efficacy of an optimal exam between ultrasound versus abbreviated MRI for HCC screening in NAFLD cirrhosis: a prospective study. Aliment Pharmacol Ther. 2022;55:820–827. doi: 10.1111/apt.16844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emhmed Ali S, Nguyen MH. Sarcopenic obesity in non-alcoholic fatty liver disease-the union of two culprits. Life (Basel) 2021;11:119. doi: 10.3390/life11020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park J, Lee EY, Li J, Jun MJ, Yoon E, Ahn SB, et al. NASH/liver fibrosis prevalence and incidence of nonliver comorbidities among people with NAFLD and Incidence of NAFLD by metabolic comorbidities: lessons from South Korea. Dig Dis. 2021;39:634–645. doi: 10.1159/000514953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ampofo AG, Boateng EB. Beyond 2020: modelling obesity and diabetes prevalence. Diabetes Res Clin Pract. 2020;167:108362. doi: 10.1016/j.diabres.2020.108362. [DOI] [PubMed] [Google Scholar]
- 13.Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440–2450. doi: 10.1056/NEJMsa1909301. [DOI] [PubMed] [Google Scholar]
- 14.Yong JN, Lim WH, Ng CH, Tan DJH, Xiao J, Tay PWL, et al. Outcomes of nonalcoholic steatohepatitis after liver transplantation: an updated meta-analysis and systematic review. Clin Gastroenterol Hepatol. 2021 Nov 18; doi: 10.1016/j.cgh.2021.11.014. doi: [DOI] [PubMed] [Google Scholar]
- 15.Muthiah MD, Sanyal AJ. Current management of non-alcoholic steatohepatitis. Liver Int. 2020;40 Suppl 1(Suppl 1):89–95. doi: 10.1111/liv.14355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao J, Lim LKE, Ng CH, Tan DJH, Lim WH, Ho CSH, et al. Is fatty liver associated with depression? A meta-analysis and systematic review on the prevalence, risk factors, and outcomes of depression and non-alcoholic fatty liver disease. Front Med (Lausanne) 2021;8:691696. doi: 10.3389/fmed.2021.691696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polanco-Briceno S, Glass D, Stuntz M, Caze A. Awareness of nonalcoholic steatohepatitis and associated practice patterns of primary care physicians and specialists. BMC Res Notes. 2016;9:157. doi: 10.1186/s13104-016-1946-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Nguyen MH. Non-alcoholic fatty liver disease (NAFLD) in Asia-more efforts are needed. Liver Int. 2020;40:3144. doi: 10.1111/liv.14657. [DOI] [PubMed] [Google Scholar]
- 19.Lazarus JV, Mark HE, Villota-Rivas M, Palayew A, Carrieri P, Colombo M, et al. The global NAFLD policy review and preparedness index: are countries ready to address this silent public health challenge? J Hepatol. 2022;76:771–780. doi: 10.1016/j.jhep.2021.10.025. [DOI] [PubMed] [Google Scholar]
- 20.Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537–2564. doi: 10.1016/j.cell.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 22.Eslam M, Sanyal AJ, George J, Sanyal A, Neuschwander-Tetri B, Tiribelli C, et al. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 23.Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14:889–919. doi: 10.1007/s12072-020-10094-2. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen VH, Le MH, Cheung RC, Nguyen MH. Differential clinical characteristics and mortality outcomes in persons with NAFLD and/or MAFLD. Clin Gastroenterol Hepatol. 2021;19:2172–2181.e6. doi: 10.1016/j.cgh.2021.05.029. [DOI] [PubMed] [Google Scholar]
- 25.Zheng Q, Zou B, Wu Y, Yeo Y, Wu H, Stave CD, et al. Systematic review with meta-analysis: prevalence of hepatic steatosis, fibrosis and associated factors in chronic hepatitis B. Aliment Pharmacol Ther. 2021;54:1100–1109. doi: 10.1111/apt.16595. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez-Fuertes M, Macías J, Corma-Gómez A, Rincón P, Merchante N, Gómez-Mateos J, et al. Similar prevalence of hepatic steatosis among patients with chronic hepatitis C with and without HIV coinfection. Sci Rep. 2020;10:6736. doi: 10.1038/s41598-020-62671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:739–752. doi: 10.1016/S2468-1253(20)30077-7. [DOI] [PubMed] [Google Scholar]
- 28.Kim D, Konyn P, Sandhu KK, Dennis BB, Cheung AC, Ahmed A. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. J Hepatol. 2021;75:1284–1291. doi: 10.1016/j.jhep.2021.07.035. [DOI] [PubMed] [Google Scholar]
- 29.Huang Q, Zou X, Wen X, Zhou X, Ji L. NAFLD or MAFLD: which has closer association with all-cause and cause-specific mortality?-results from NHANES III. Front Med (Lausanne) 2021;8:693507. doi: 10.3389/fmed.2021.693507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee H, Lee YH, Kim SU, Kim HC. Metabolic dysfunction-associated fatty liver disease and incident cardiovascular disease risk: a nationwide cohort study. Clin Gastroenterol Hepatol. 2021;19:2138–2147.e10. doi: 10.1016/j.cgh.2020.12.022. [DOI] [PubMed] [Google Scholar]
- 31.Liang Y, Chen H, Liu Y, Hou X, Wei L, Bao Y, et al. Association of MAFLD with diabetes, chronic kidney disease, and cardiovascular disease: a 4.6-year cohort study in China. J Clin Endocrinol Metab. 2022;107:88–97. doi: 10.1210/clinem/dgab641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoneda M, Yamamoto T, Honda Y, Imajo K, Ogawa Y, Kessoku T, et al. Risk of cardiovascular disease in patients with fatty liver disease as defined from the metabolic dysfunction associated fatty liver disease or nonalcoholic fatty liver disease point of view: a retrospective nationwide claims database study in Japan. J Gastroenterol. 2021;56:1022–1032. doi: 10.1007/s00535-021-01828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee YB, Ha Y, Chon YE, Kim MN, Lee JH, Park H, et al. Association between hepatic steatosis and the development of hepatocellular carcinoma in patients with chronic hepatitis B. Clin Mol Hepatol. 2019;25:52–64. doi: 10.3350/cmh.2018.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Chaung KT, Nguyen P, Le AK, Hoang J, Nguyen MH. High rate of progression to cirrhosis in chronic hepatitis B (CHB) patients with non-alcoholic fatty liver (FL) Gastroenterology. 2017;152:S1081–S1082. [Google Scholar]
- 35.Zheng KI, Sun DQ, Jin Y, Zhu PW, Zheng MH. Clinical utility of the MAFLD definition. J Hepatol. 2021;74:989–991. doi: 10.1016/j.jhep.2020.12.016. [DOI] [PubMed] [Google Scholar]
- 36.Wang MF, Wan B, Wu YL, Huang JF, Zhu YY, Li YB. Clinic-pathological features of metabolic associated fatty liver disease with hepatitis B virus infection. World J Gastroenterol. 2021;27:336–344. doi: 10.3748/wjg.v27.i4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Omary A, Byth K, Weltman M, George J, Eslam M. The importance and impact of recognizing metabolic dysfunction-associated fatty liver disease in patients with chronic hepatitis C. J Dig Dis. 2022;23:33–43. doi: 10.1111/1751-2980.13071. [DOI] [PubMed] [Google Scholar]
- 38.van Kleef LA, Choi HSJ, Brouwer WP, Hansen BE, Patel K, de Man RA, et al. Metabolic dysfunction-associated fatty liver disease increases risk of adverse outcomes in patients with chronic hepatitis B. JHEP Rep. 2021;3:100350. doi: 10.1016/j.jhepr.2021.100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin YP, Lin SH, Wang CC, Lin CC, Chen DW, Chuang CH, et al. Personalized medicine impact of MAFLD on HBV-related stage 0/A hepatocellular carcinoma after curative resection impact of MAFLD on HBV-related. J Pers Med. 2021;11:684. doi: 10.3390/jpm11080684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi HSJ, Brouwer WP, Zanjir WMR, de Man RA, Feld JJ, Hansen BE, et al. Nonalcoholic steatohepatitis is associated with liver-related outcomes and all-cause mortality in chronic hepatitis B. Hepatology. 2020;71:539–548. doi: 10.1002/hep.30857. [DOI] [PubMed] [Google Scholar]
- 41.Kim MN, Han K, Yoo J, Hwang SG, Ahn SH. Increased risk of hepatocellular carcinoma and mortality in chronic viral hepatitis with concurrent fatty liver. Aliment Pharmacol Ther. 2022;55:97–107. doi: 10.1111/apt.16706. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Yang HI, Yeh ML, Le MH, Le AK, Yeo YH, et al. Association between fatty liver and cirrhosis, hepatocellular carcinoma, and hepatitis B surface antigen seroclearance in chronic hepatitis B. J Infect Dis. 2021;224:294–302. doi: 10.1093/infdis/jiaa739. [DOI] [PubMed] [Google Scholar]
- 43.Mak LY, Hui RW, Fung J, Liu F, Wong DK, Cheung KS, et al. Diverse effects of hepatic steatosis on fibrosis progression and functional cure in virologically quiescent chronic hepatitis B. J Hepatol. 2020;73:800–806. doi: 10.1016/j.jhep.2020.05.040. [DOI] [PubMed] [Google Scholar]
- 44.Chu CM, Lin DY, Liaw YF. Does increased body mass index with hepatic steatosis contribute to seroclearance of hepatitis B virus (HBV) surface antigen in chronic HBV infection? Int J Obes (Lond) 2007;31:871–875. doi: 10.1038/sj.ijo.0803479. [DOI] [PubMed] [Google Scholar]
- 45.Chu CM, Lin DY, Liaw YF. Clinical and virological characteristics post HBsAg seroclearance in hepatitis B virus carriers with hepatic steatosis versus those without. Dig Dis Sci. 2013;58:275–281. doi: 10.1007/s10620-012-2343-9. [DOI] [PubMed] [Google Scholar]
- 46.Oh JH, Lee HW, Sinn DH, Park JY, Kim BK, Kim SU, et al. Controlled attenuation parameter value and the risk of hepatocellular carcinoma in chronic hepatitis B patients under antiviral therapy. Hepatol Int. 2021;15:892–900. doi: 10.1007/s12072-021-10205-7. [DOI] [PubMed] [Google Scholar]
- 47.Mak LY, Hui RW, Fung J, Liu F, Wong DK, Li B, et al. Reduced hepatic steatosis is associated with higher risk of hepatocellular carcinoma in chronic hepatitis B infection. Hepatol Int. 2021;15:901–911. doi: 10.1007/s12072-021-10218-2. [DOI] [PubMed] [Google Scholar]
- 48.Wong SW, Chan WK, Mohamed R. Fatty liver is associated with advanced fibrosis but does not predict adverse outcomes in patients with chronic hepatitis B. J Viral Hepat. 2020;27:1297–1305. doi: 10.1111/jvh.13361. [DOI] [PubMed] [Google Scholar]
- 49.Targher G, Corey KE, Byrne CD, Roden M. The complex link between NAFLD and type 2 diabetes mellitus - mechanisms and treatments. Nat Rev Gastroenterol Hepatol. 2021;18:599–612. doi: 10.1038/s41575-021-00448-y. [DOI] [PubMed] [Google Scholar]
- 50.Kushner RF, Ryan DH. Assessment and lifestyle management of patients with obesity: clinical recommendations from systematic reviews. JAMA. 2014;312:943–952. doi: 10.1001/jama.2014.10432. [DOI] [PubMed] [Google Scholar]
- 51.Yilmaz Y, Byrne CD, Musso G. A single-letter change in an acronym: signals, reasons, promises, challenges, and steps ahead for moving from NAFLD to MAFLD. Expert Rev Gastroenterol Hepatol. 2021;15:345–352. doi: 10.1080/17474124.2021.1860019. [DOI] [PubMed] [Google Scholar]
- 52.Eslam M, Ahmed A, Després JP, Jha V, Halford JCG, Wei Chieh JT, et al. Incorporating fatty liver disease in multidisciplinary care and novel clinical trial designs for patients with metabolic diseases. Lancet Gastroenterol Hepatol. 2021;6:743–753. doi: 10.1016/S2468-1253(21)00132-1. [DOI] [PubMed] [Google Scholar]
- 53.Choi JH, Sohn W, Cho YK. The effect of moderate alcohol drinking in nonalcoholic fatty liver disease. Clin Mol Hepatol. 2020;26:662–669. doi: 10.3350/cmh.2020.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.GBD 2016 Alcohol Collaborators Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392:1015–1035. doi: 10.1016/S0140-6736(18)31310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crabb DW, Im GY, Szabo G, Mellinger JL, Lucey MR. Diagnosis and treatment of alcohol-associated liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71:306–333. doi: 10.1002/hep.30866. [DOI] [PubMed] [Google Scholar]
- 56.Cherpitel CJ, Ye Y, Stockwell T, Vallance K, Chow C. Recall bias across 7 days in self-reported alcohol consumption prior to injury among emergency department patients. Drug Alcohol Rev. 2018;37:382–388. doi: 10.1111/dar.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quek J, Ng CH, Tang ASP, Chew N, Chan M, Khoo CM, et al. Metabolic associated fatty liver disease increases the risk of systemic complications and mortality. A meta-analysis and systematic review of 12620736 individuals. Endocr Pract. 2022;28:667–672. doi: 10.1016/j.eprac.2022.03.016. [DOI] [PubMed] [Google Scholar]
- 58.Canbay A, Kachru N, Haas JS, Sowa JP, Meise D, Ozbay AB. Patterns and predictors of mortality and disease progression among patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2020;52:1185–1194. doi: 10.1111/apt.16016. [DOI] [PubMed] [Google Scholar]
- 59.Le MH, Yeo YH, Cheung R, Wong VW, Nguyen MH. Ethnic influence on nonalcoholic fatty liver disease prevalence and lack of disease awareness in the United States, 2011-2016. J Intern Med. 2020;287:711–722. doi: 10.1111/joim.13035. [DOI] [PubMed] [Google Scholar]
- 60.Shiha G, Alswat K, Al Khatry M, Sharara AI, Örmeci N, Waked I, et al. Nomenclature and definition of metabolic-associated fatty liver disease: a consensus from the Middle East and North Africa. Lancet Gastroenterol Hepatol. 2021;6:57–64. doi: 10.1016/S2468-1253(20)30213-2. [DOI] [PubMed] [Google Scholar]
- 61.Nan Y, An J, Bao J, Chen H, Chen Y, Ding H, et al. The Chinese Society of Hepatology position statement on the redefinition of fatty liver disease. J Hepatol. 2021;75:454–461. doi: 10.1016/j.jhep.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 62.Shaltout I, Alkandari H, Fouad Y, Hamed AE. Arabic Association for the Study of Diabetes and Metabolism (AASD) endorsing the MAFLD definition of fatty liver disease. J Hepatol. 2022;76:739–740. doi: 10.1016/j.jhep.2021.11.027. [DOI] [PubMed] [Google Scholar]
- 63.Mendez-Sanchez N, Arrese M, Gadano A, Oliveira CP, Fassio E, Arab JP, et al. The Latin American Association for the Study of the Liver (ALEH) position statement on the redefinition of fatty liver disease. Lancet Gastroenterol Hepatol. 2021;6:65–72. doi: 10.1016/S2468-1253(20)30340-X. [DOI] [PubMed] [Google Scholar]
- 64.Méndez-Sánchez N, Bugianesi E, Gish RG, Lammert F, Tilg H, Nguyen MH, et al. Global multi-stakeholder endorsement of the MAFLD definition. Lancet Gastroenterol Hepatol. 2022;7:388–390. doi: 10.1016/S2468-1253(22)00062-0. [DOI] [PubMed] [Google Scholar]
- 65.Singh SP, Anirvan P, Reddy KR, Conjeevaram HS, Marchesini G, Rinella ME, et al. Non-alcoholic fatty liver disease: not time for an obituary just yet! J Hepatol. 2021;74:972–974. doi: 10.1016/j.jhep.2020.10.015. [DOI] [PubMed] [Google Scholar]
- 66.Younossi ZM, Rinella ME, Sanyal AJ, Harrison SA, Brunt EM, Goodman Z, et al. From NAFLD to MAFLD: implications of a premature change in terminology. Hepatology. 2021;73:1194–1198. doi: 10.1002/hep.31420. [DOI] [PubMed] [Google Scholar]
- 67.Francque SM, Bedossa P, Ratziu V, Anstee QM, Bugianesi E, Sanyal AJ, et al. A randomized, controlled trial of the Pan-PPAR agonist lanifibranor in NASH. N Engl J Med. 2021;385:1547–1558. doi: 10.1056/NEJMoa2036205. [DOI] [PubMed] [Google Scholar]
- 68.Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184–2196. doi: 10.1016/S0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]
- 69.Loomba R, Adams LA. Advances in non-invasive assessment of hepatic fibrosis. Gut. 2020;69:1343–1352. doi: 10.1136/gutjnl-2018-317593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harrison SA, Ratziu V, Boursier J, Francque S, Bedossa P, Majd Z, et al. A blood-based biomarker panel (NIS4) for non-invasive diagnosis of non-alcoholic steatohepatitis and liver fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:970–985. doi: 10.1016/S2468-1253(20)30252-1. [DOI] [PubMed] [Google Scholar]
- 71.Dela Cruz AC, Bugianesi E, George J, Day CP, Liaquat H, Charatcharoenwitthaya P, et al. 379 characteristics and long-term prognosis of lean patients with nonalcoholic fatty liver disease. Gastroenterology. 2014;146:S909. [Google Scholar]
- 72.Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, et al. Risk for development of severe liver disease in lean patients with nonalcoholic fatty liver disease: a long-term follow-up study. Hepatol Commun. 2017;2:48–57. doi: 10.1002/hep4.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zou B, Yeo YH, Nguyen VH, Cheung R, Ingelsson E, Nguyen MH. Prevalence, characteristics and mortality outcomes of obese, nonobese and lean NAFLD in the United States, 1999-2016. J Intern Med. 2020;288:139–151. doi: 10.1111/joim.13069. [DOI] [PubMed] [Google Scholar]
- 74.Ito T, Ishigami M, Zou B, Tanaka T, Takahashi H, Kurosaki M, et al. The epidemiology of NAFLD and lean NAFLD in Japan: a meta-analysis with individual and forecasting analysis, 1995-2040. Hepatol Int. 2021;15:366–379. doi: 10.1007/s12072-021-10143-4. [DOI] [PubMed] [Google Scholar]
- 75.Younes R, Govaere O, Petta S, Miele L, Tiniakos D, Burt A, et al. Caucasian lean subjects with non-alcoholic fatty liver disease share long-term prognosis of non-lean: time for reappraisal of BMI-driven approach? Gut. 2022;71:382–390. doi: 10.1136/gutjnl-2020-322564. [DOI] [PubMed] [Google Scholar]
- 76.Wong VW, Chan WK, Chitturi S, Chawla Y, Dan YY, Duseja A, et al. Asia-Pacific Working Party on non-alcoholic fatty liver disease guidelines 2017-part 1: definition, risk factors and assessment. J Gastroenterol Hepatol. 2018;33:70–85. doi: 10.1111/jgh.13857. [DOI] [PubMed] [Google Scholar]
- 77.Chitturi S, Wong VW, Chan WK, Wong GL, Wong SK, Sollano J, et al. The Asia-Pacific Working Party on non-alcoholic fatty liver disease guidelines 2017-part 2: management and special groups. J Gastroenterol Hepatol. 2018;33:86–98. doi: 10.1111/jgh.13856. [DOI] [PubMed] [Google Scholar]
- 78.European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD) European Association for the Study of Obesity (EASO) EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]