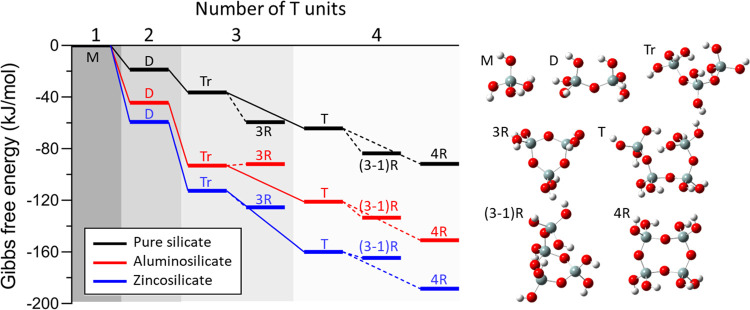
Figure 5.
Formation Gibbs free energies of oligomers (up to n = 4, where n is the number of tetrahedral species, i.e., Si, Al, or Zn) calculated using DFT (M = monomer, D = dimer, Tr = trimer, 3R = 3-membered ring, T = tetramer, (3-1)R = 3-membered ring with one acyclic silicon, and 4R = 4-membered ring). Oligomer growth via condensation is represented by solid lines, and cyclization is represented by dotted lines. Aluminosilicate and zincosilicate isomers with the most exothermic formation energy values are reported. Optimized molecular structures of pure-silicate oligomers are provided with color-coded balls representing silicon (gray), oxygen (red), and hydrogen (white) atoms. Additional details are given in Figures S11 and S12.