Fig. 4.
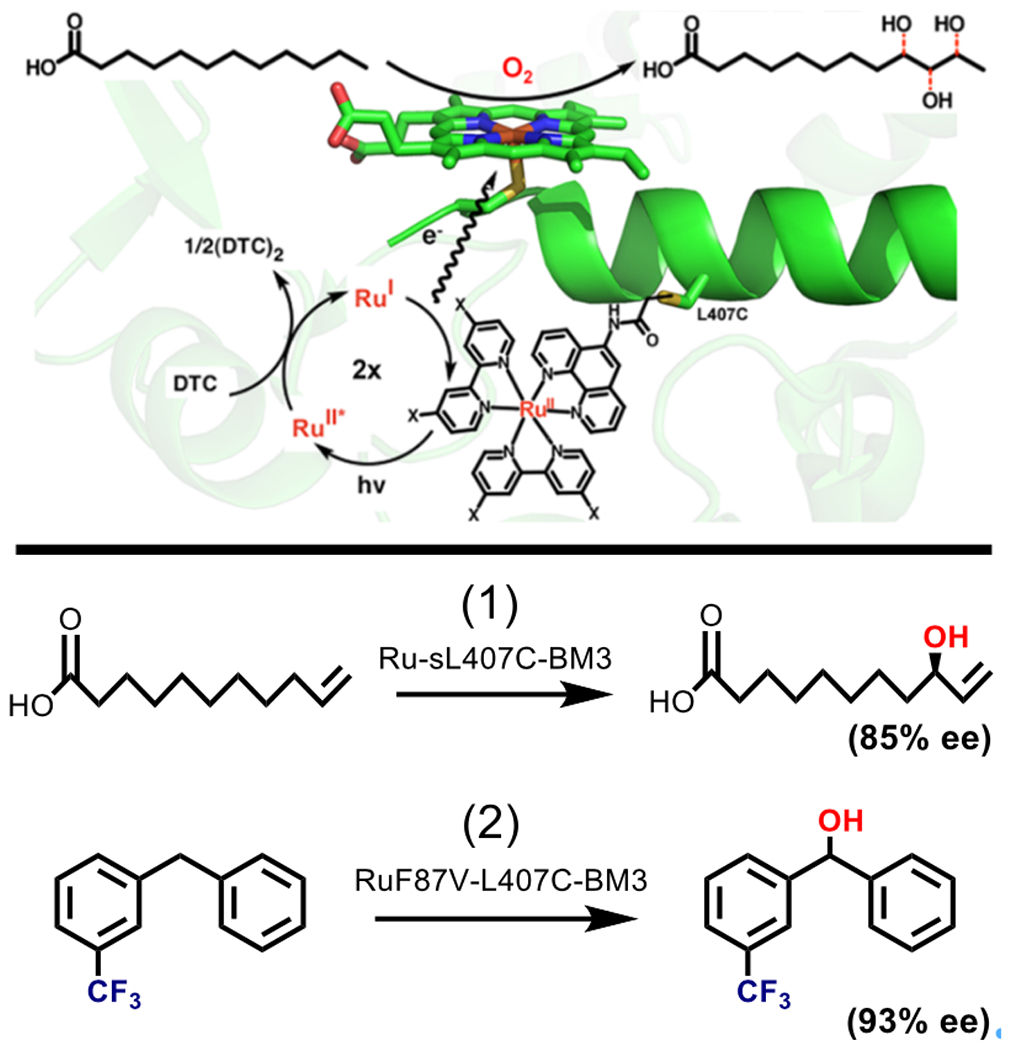
Top: Diagram showing the Ru(II) diimine sensitizer (X = H, OMe) attached via 407C in close proximity to the P450 BM3 active site. Excitation of the Ru (II) sensitizer generates an Ru (I) species that donates an electron to the heme, fueling the production of three monohydroxylated products of lauric acid. Adapted with permission from Tran N, Nguyen D, Dwaraknath S, Mahadevan S, Chavez G, Nguyen A, Dau T, Mullen S, Nguyen T-A, Cheruzel L (2013) J. Am. Chem. Soc. 135, 14484–14487. Copyright (2013) American Chemical Society. Bottom: Scheme showing other reactions catalyzed by mutants on the Ru-L407C-BM3 background. (1) The light driven hydroxylation of 10-undecenoic acid is catalyzed by Ru-sL407C-BM3 with 85% enantiomeric excess. (2) L407C mutants have also been used to hydroxylate trifluoromethylated substrates, for example meta-trifluoromethylated diphenylmethane was hydroxylated with 93% ee by RuF87V-L407C-BM3.
