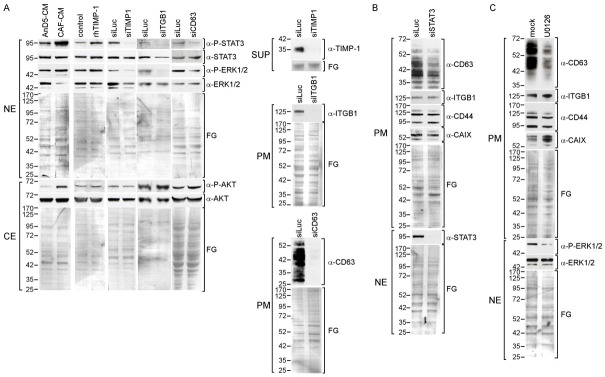
Figure 3.
TIMP-1 activates STAT3 and ERK1/2 in concert with CD63 and ITGB1. (A–C) Western blot analyses of protein extracts from AnD5 cells. (A) The activities of STAT3, ERK1/2 and AKT proteins in AnD5 cells were determined by their phosphorylation statuses by using nuclear extracts (NE) or cytosolic extracts (CE) in the presence of AnD5-CM or CAF-CM, in the presence or absence of rhTIMP-1 or after transfection with siRNAs as indicated. The knock-down effects of siTIMP1, siITGB1 and siCD63 were confirmed by analyzing the supernatants (sup) or plasma membrane extracts (PM) for the abundances of TIMP-1, ITGB1 and CD63, respectively. To study the effects of AnD5-CM, CAF-CM and siRNAs, cells were incubated for three days, and cells were exposed to rhTIMP-1 for one day. (B,C) Effect of siSTAT3 and U0126 on the abundances of several membrane proteins as indicated. Protein extractions were performed after a three-day-treatment with control siRNA siLuc or siSTAT3 (B) or after a one-day-exposure to U0126 or mock (C). The effect of siSTAT3 on STAT3 expression (B) and of U0126 on ERK1/2 phosphorylation (C) was determined by analyzing nuclear extracts. To show equal protein loading, the membranes were stained by Fast Green (FG).