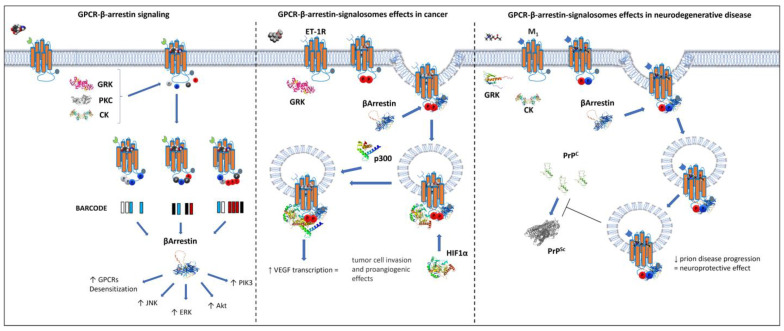
Figure 2.
GPCR/β-arrestin-dependent signaling pathway. GPCRs are illustrated as orange transmembrane proteins whose carboxyl groups are shown as gray dots and their N-terminal groups as differently shaped symbols. GPCR phosphorylation processes can take place through the action of GPCR kinases (GRKs) or casein kinase (CKs), represented with red and blue dots, respectively. β-arrestin functions are to reduce GPCR coupling to G proteins, favor the internalization of the receptor in endosomes and signal through the GPCR-β-arrestin complex to promote JNK, ERK, phosphoinositide 3 kinase (PIK3), and Akt signaling. Different degrees of GPCR phosphorylation affect the interaction between receptor and β-arrestin, thus initiating preferential intracellular responses such as β-arrestin mediating ERK, JNK, or GPCR desensitization (barcode theory). In cancer cells, these GPCR–β-arrestin-dependent multiprotein complexes interact with signaling proteins involved in gene transcription: in ovarian cancer, the activation of ET-1R promotes the interaction between β-arr1/p300 and HIF-1α, enhancing the transcription of genes, such as ET-1 and VEGF, required for tumor cell invasion and proangiogenic effects. Meanwhile, in neurodegenerative disease, GPCR–β-arrestin signalosomes exert a crucial neuroprotective effect as shown in the right part of the figure, where the complexed, internalized muscarinic M1 receptor signaling reduces the accumulation of misfolded prion protein (PrPsc).