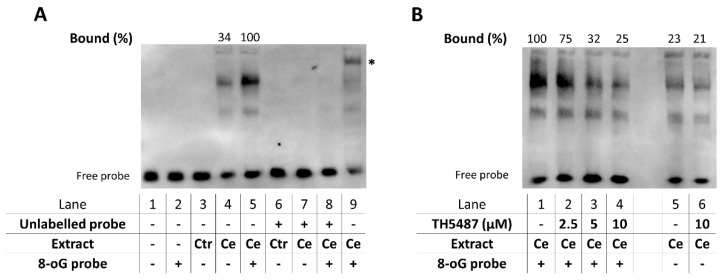
Figure 7.
OGG1 inhibition reduces the amount of active NF-κB in AP and decreases NF-κB-binding to 8-oxoG-containing response elements. (A) Pancreatic nuclear extracts were prepared from mice treated with saline (Ctr) or cerulein (Ce). EMSA was performed by incubating the extracts with native or 8-oxoG modified NFκB-response element containing oligonucleotide probes. A mobility shift of the native probe confirmed that the Ce nuclear extracts contained active NF-κB (lane 4), whereas the Ctr extract caused no shift (lane 3). Nearly 3-fold increase was detected in NF-κB binding when a guanine by the response element in the probe was replaced with 8-oxoG (lane 5 vs. 4). The assay specificity was confirmed by competing off binding with an excess of unlabeled probe (lane 7–8) and by supershifting the probe with an anti-p65 antibody added to the Ce extract prior to the EMSA (lane 9, asterisk). (B) TH5487 when added to the Ce extract before the DNA, decreased NF-κB binding to 8-oxoG containing probes in a dose-dependent manner (lanes 1–4). TH5487 markedly decreased NF-κB DNA occupancy in the presence of 8-oxoG next to the response element (lane 4) but had no effect in the absence of 8-oxoG (lane 6). Data are representative of three experiments. Binding occupancies were calculated from band intensities using the Image J software. Relative band intensities were compared to the Ce extract incubated with the 8-oxoG probe, which was taken as 100% (lane 5 and lane 1 in panels A and B, respectively).