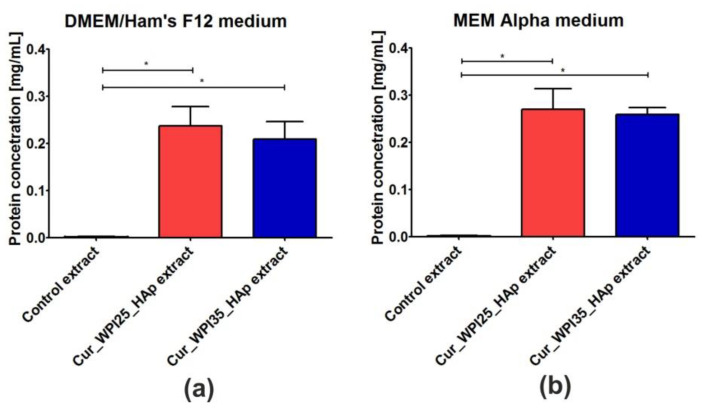
Figure 4.
The ability of Cur_WPI25_HAp and Cur_WPI35_HAp biomaterials to release protein in a liquid environment. The extracts from biomaterials were prepared according to ISO 10993-12:2012 standard: Biological evaluation of medical devices—Part 12: Sample preparation and reference materials [33]. The biomaterial samples were immersed in serum-free culture media intended for osteoblast culture (DMEM/Ham F12 medium (a) or MEM alpha medium (b)) in the proportion equal to 0.1 g of biomaterials per 1 mL of media. Control extracts were obtained by incubation of culture media in non-cytotoxic polystyrene cell culture plate. The biomaterials were incubated at 37 °C for 24 h. The unpaired Student’s t-test was performed in order to determine statistical differences between samples, p < 0.05: * Significantly different results between concentration of protein in extracts obtained from Cur_WPI25_HAp and Cur_WPI35_HAp biomaterials and protein content in culture media.