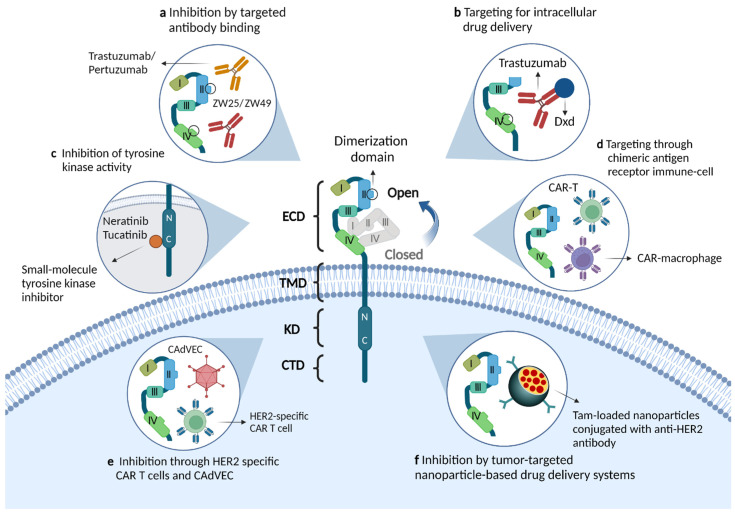
Figure 1.
Molecular structure of the ERBB2/HER2 receptor and the mechanism of action of novel therapeutic agents. (a) Monoclonal antibodies that target binding to the extracellular structural domain of HER2, including single-epitope monoclonal antibodies (trastuzumab, pertuzumab) and bispecific HER2 antibodies (ZW25, ZW49). The antitumor effects of these drugs are mediated through a variety of mechanisms: inhibition of downstream signaling pathways, involvement in antibody-dependent cellular cytotoxicity or inhibition of receptor dimerization. (b) Antibody-drug conjugates targeting HER2, such as trastuzumab emtansine and trastuzumab deruxtecan, release and deliver cytotoxic drugs while binding antibodies and thus exert anti-tumor effects. (c) Small molecule inhibitors, such as neratinib and tucatinib, inhibit activation of the PI3K pathway by binding to the tyrosine-kinase domain of the HER2 receptor. (d,e) HER2-targeted immunotherapies, such as HER2-specific chimeric antigen receptor immune cells or modified oncolytic virus, enhance immune cell activity and stimulate anti-tumor immune responses. (f) Tumor-targeting nanoparticles, such as nanodelivery systems with internal encapsulation of tamoxifen and external loading of anti-HER2 antibodies, can efficiently kill HER2 overexpressing breast cancer cells by penetrating the tumor microenvironment.