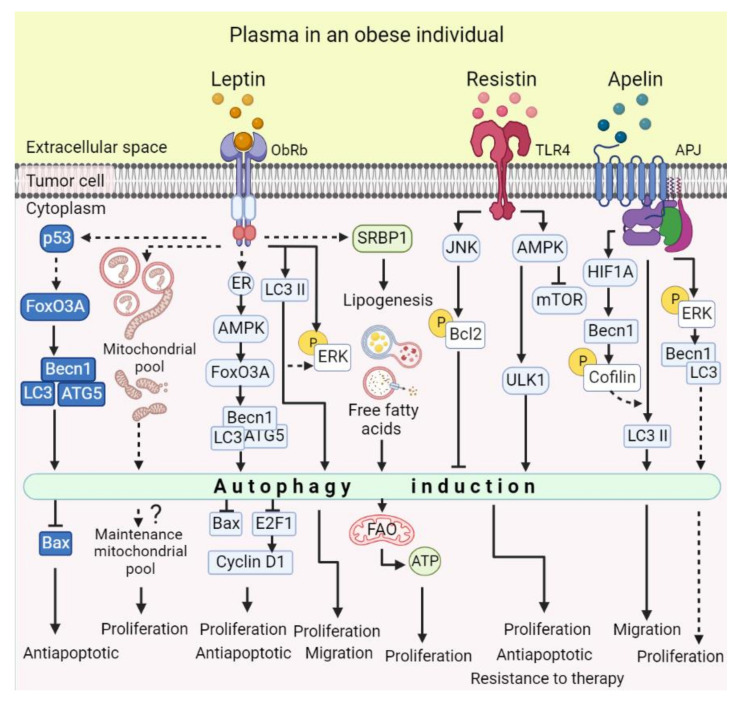
Figure 4.
Molecular mechanisms of the effect of adipokines on autophagy and tumor cell malignancy. Different mechanisms involved in the induction of autophagy by leptin have been described. Leptin-induced autophagy has been shown to regulate proteins involved in proliferation and apoptosis, such as cyclin D1 and Bax. The autophagy-mediated regulation of Bax involves the p53/FoxO3A axis, which transcriptionally regulates autophagy-related proteins. Specifically, in ER-positive breast cancer cells, leptin-induced autophagy is ER-dependent, involves AMPK activation, and regulates Bax and Cyclin D1 levels. Leptin-induced autophagy is implicated in cellular migration through the regulation of ERK phosphorylation. Additionally, leptin-induced autophagy could be a mechanism for maintaining the mitochondrial pool, which would influence energetic metabolism. In this sense, leptin-induced autophagy is involved in the degradation of intracellular fat droplets, which provide free fatty acids that are oxidized and improve cancer cell metabolism. The first evidence for the role of resistin in autophagy demonstrated that resistin induces autophagy by decreasing the phosphorylation of mTOR and ULK1, and upregulating p-AMPK. Another important signaling molecule involved in resistin-induced autophagy was JNK kinase, which mediated the phosphorylation of Bcl-2. Bcl-2 phosphorylation is known to lead to its dissociation from Beclin1, a required step in the autophagy process. On the other hand, apelin/APJ increases autophagy and promotes Becn1 expression via HIF1A. Interestingly, Beclin 1 regulates cofilin phosphorylation, which was important for apelin/autophagy-induced migration in lung adenocarcinoma. Additionally, it has been demonstrated that apelin-induced Becn1 and LC3 protein levels are dependent on the phosphorylation of ERK. This suggests an essential role for ERK in regulating apelin-induced autophagy proteins. Solid line: demonstrated mechanism; dashed lines: unknown mechanisms.