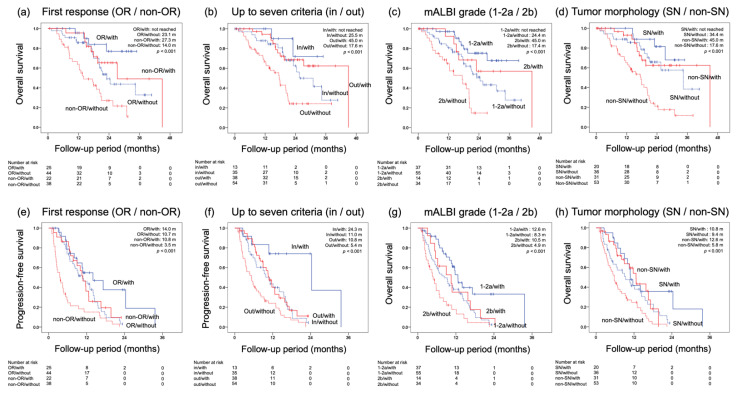
Figure 3.
Comparison of overall survival (OS) and progression-free survival (PFS) by four factors, focusing on transarterial chemoembolization (TACE) in combination. (a) OS by the response at the first evaluation and TACE combination (objective response (OR)/with TACE not-reached, OR/without TACE 23.1 months, non-OR/with TACE 27.3 months, non-OR/without TACE 14.0 months, p < 0.001). (b) OS by up to seven criteria and TACE combination (up to seven criteria in/with TACE not-reached, up to seven criteria in/without TACE 25.5 months, up to seven criteria out/with TACE 45.0 months, up to seven criteria out/without TACE 17.6 months, p < 0.001). (c) OS by tumor morphology and TACE combination (simple nodular [SN]/with TACE not-reached, SN/without TACE 34.4 months, non-SN/with TACE 45.0 months, non-SN/without TACE 17.6 months, p < 0.001). (d) OS by modified albumin–bilirubin (mALBI) grade and TACE combination (mALBI 1–2a/with TACE not-reached, mALBI 1–2a/without TACE 24.4 months, mALBI 2b/with TACE 45.0 months, mALBI 2b/without TACE 17.4 months, p < 0.001). (e) PFS by the response at the first evaluation and TACE combination (OR/with TACE 14.0 months, OR/without TACE 10.7 months, non-OR/with TACE 10.8 months, non-OR/without TACE 3.5 months, p < 0.001). (f) PFS by up to seven criteria and TACE combination (up to seven criteria in/with TACE 24.3 months, up to seven criteria in/without TACE 11.0 months, up to seven criteria out/with TACE 10.8 months, up to seven criteria out/without TACE 5.4 months, p < 0.001). (g) PFS by mALBI grade and TACE combination (mALBI 1–2a/with TACE 12.6 months, mALBI 1–2a/without TACE 8.3 months, mALBI 2b/with TACE 10.5 months, mALBI 2b/without TACE 4.9 months, p < 0.001). (h) PFS by tumor morphology and TACE combination (SN/with TACE 10.8 months, SN/without TACE 9.4 months, non-SN/with TACE 12.6 months, non-SN/without TACE 5.8 months, p < 0.001).