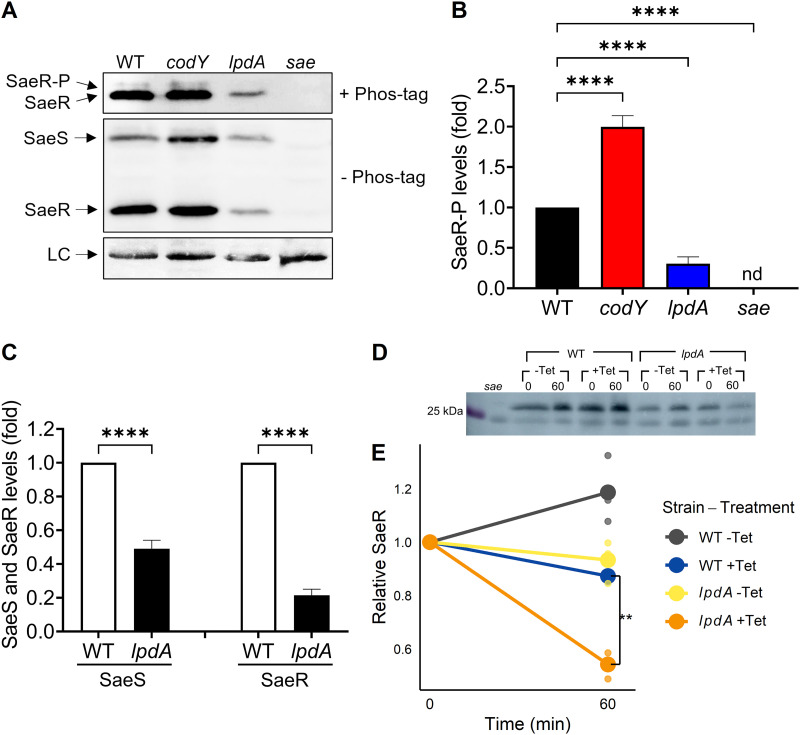
FIG 7.
LpdA is required for promoting the state of phosphorylated SaeR in vivo. (A) Phos-tag Western blot (top) or Western blot (middle and bottom) analysis of crude extracts prepared from wild-type (WT), codY, lpdA, and sae USA300 strains grown in TSB medium for 5 h using antibodies recognizing SaeR. LC, loading control. (B) Levels of SaeR~P relative to SaeR~P from wild type. nd, not detected. (C) Levels of SaeS and SaeR from the lpdA mutant relative to those from the wild type were determined by SDS-PAGE and western blot analysis. All data are representative of three independent experiments, which produced similar results. One-way ANOVA with Tukey's post test was used for statistical analysis comparing SaeR~P levels. n > 3. ****, P < 0.0001. (D and E) Strains lacking the entire sae operon were complemented with saeRS under the control of the constitutive P3 promoter, in an otherwise wild-type (WT) and lpdA background; sae refers to the uncomplemented strain. Strains were grown for 4 h before supplementation with 15 μg mL−1 tetracycline; samples were removed for Western blotting at the beginning and end of a 1-h time course (0 and 60 min). Blots were normalized by total protein concentration. −Tet, no tetracycline; +Tet, tetracycline supplementation. (D) Representative blot of WT, lpdA, and sae whole-cell lysates. (E) Relative decrease in SaeR protein levels during 1-h translation blockade using tetracycline treatment. Lines connect the mean relative abundances at the beginning and end of the time course, and dots show individual biological replicates. n = 3. *, P < 0.05; **, P < 0.01, using ANOVA followed by Tukey’s HSD test.