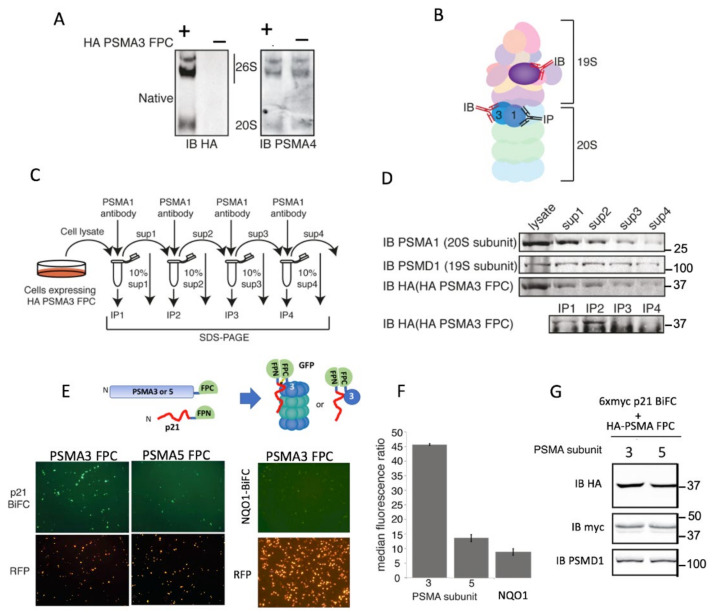
Figure 2.
PSMA3 interacts with p21 in the cells. (A) U2OS cells stably expressing PSMA3–FPC. Cell lysates were enriched with proteasomes by ultracentrifugation and loaded on native gel. The membrane was probed with antibody against either HA-tag or the endogenous subunit PSMA4. (B) Schematic description of the antibodies used against the different 26S proteasome subunits for the described co-immunoprecipitation experiments to demonstrate the possible incorporation of a chimeric PSMA3 subunit into proteasomes. The endogenous PSMA1 subunit was first immunoprecipitated and the level of the co-immunoprecipitated subunits was monitored using antibodies to detect the endogenous PSMD1, a subunit of the 19S proteasome, and anti-HA to detect the chimeric PSMA3. (C) The schematic description of the experimental strategy of serial consecutive immunoprecipitation steps. (D) The results obtained from each of the steps described in Panel C. HEK293 cells expressing HA–PSMA3–FPC were harvested 24 h post-transfection. Cells’ lysate was subjected to four subsequent immunoprecipitations of proteasomes via the endogenous PSMA1 subunit. Ten percent of cell lysate was kept for analysis after each immunoprecipitation. (E) Cells were transfected with either PSMA3–FPC or PSMA5–FPC together with p21 FPN (see scheme). We also transfected the cells with H2B–RFP, which provides RFP labeling of the transfected cells’ nuclei. Successful BiFC using fluorescent microscopy, 20× objective 48 h post-transfection. (F) Intensities of at least 10,000 cells for each PSMA–p21 and PSMA–NQO1 combination were recorded by flow cytometry. Standard deviation bars represent two biological replica. (G) Expression level of the proteins in the cells was examined.