FIG 3.
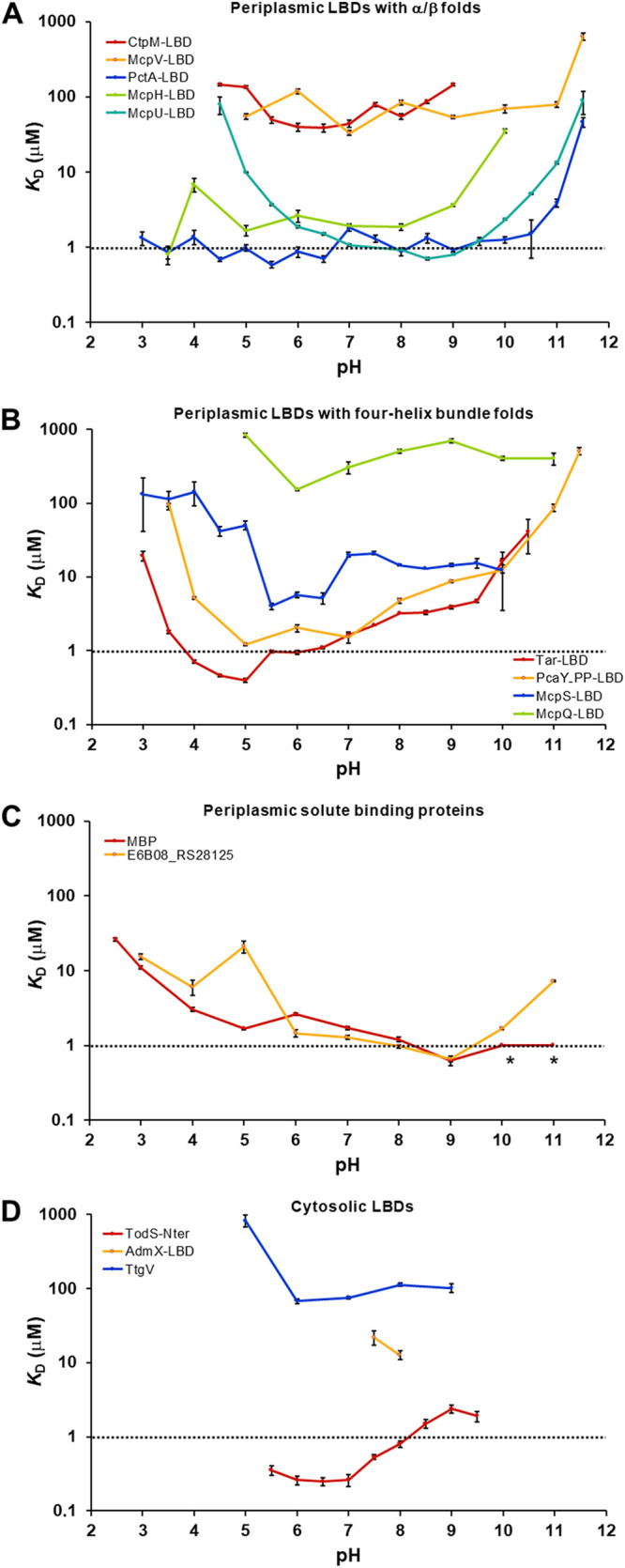
The pH dependence of ligand-binding for different proteins. The dissociation constants (KD) at different pH values are shown for periplasmic LBDs with α/β folds (A) four-helixbundle folds (B), periplasmic solute-binding proteins (C), and cytosolic LBDs (D). The titration curves and the derived thermodynamic parameters are shown in Fig. S1 to S3 and Table S2. The following ligands were used for the binding studies: CtpM: L-malate, McpV: propionate, PctA: l-alanine, McpH: adenine, McpU: putrescine, Tar: L-aspartate, PcaY_PP: quinate, McpS: L-malate, McpQ: citrate, MBP: D-maltose, E6B08_RS28125: l-ornithine, TodS: toluene, AdmX: indole-3-pyruvic acid, TtgV: benzonitrile. The asterisks indicate high-affinity binding, but data analysis with different models failed.
