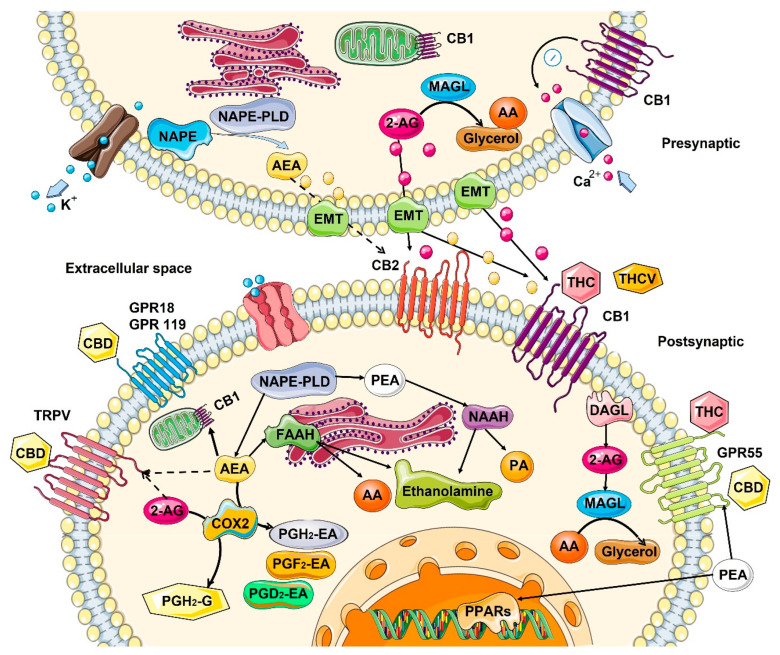
Figure 1.
Biosynthesis, degradation, and interaction of endocannabinoids with cannabinoid receptors. Biosynthesis and the inactivation of the two endogenous lipid messengers, such as endocannabinoids N-Arachidonoylethanolamine or anandamide (AEA), 2-arachidonoylglycerol (2-AG), and N-palmitoylethanolamide (PEA) act on cannabinoid receptors. AEA and 2-AG are typically released on demand from membrane lipids. AEA synthesized from N-arachidonoyl-phosphatidylethanolamines (NAPE) via the activity of N-acyl-phosphatidylethanolamine-hydrolyzing phospholipase D (NAPE-PLD) and hydrolyzed by fatty acid amide hydrolase (FAAH) to ethanolamine and arachidonic acid (AA). 2-AG is 2-AG can also be produced from sn-2-arachidonate-containing diacylglycerols by sn-1-acyl-2-arachidonoylglycerol lipase (DAGL), and degraded by lipase (MAGL), releasing glycerol and AA. PEA is hydrolyzed by N-acylethanolamine-hydrolyzing acid amidase (NAAA) into ethanolamine and palmitic acid (PA). Cyclooxygenase-2 (COX-2) can also oxidize anandamide and 2-AG, followed by prostaglandin synthases to produce prostamides (from anandamide) and prostaglandin-ethanolamide, PG-EA (from 2-AG). Both AEA and 2-AG move across the plasma membrane via a purported endocannabinoid membrane transporter (EMT) and target CB1 and CB2, which show an extracellular binding site. 2-AG, AEA, and PEA directly activate orphan G-protein-coupled receptors (GPR55, GPR18, GPR119), the transient receptor potential of vanilloid (TRPV) channel, and peroxisome proliferator-activated nuclear receptors (PPARs). Dashed lines denote low-affinity bindings. Phytocannabinoids Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD), and Δ9-tetrahydrocannabivarin (THCV) showed to activate cannabinoid receptors. CB1, cannabinoid receptor 1; CB2, cannabinoid receptor 2; ER, endoplasmic reticulum. This figure was created using images from Servier Medical Art Commons Attribution 3.0 Unported License (http://smart.servier.com (accessed on 4 May 2022)).