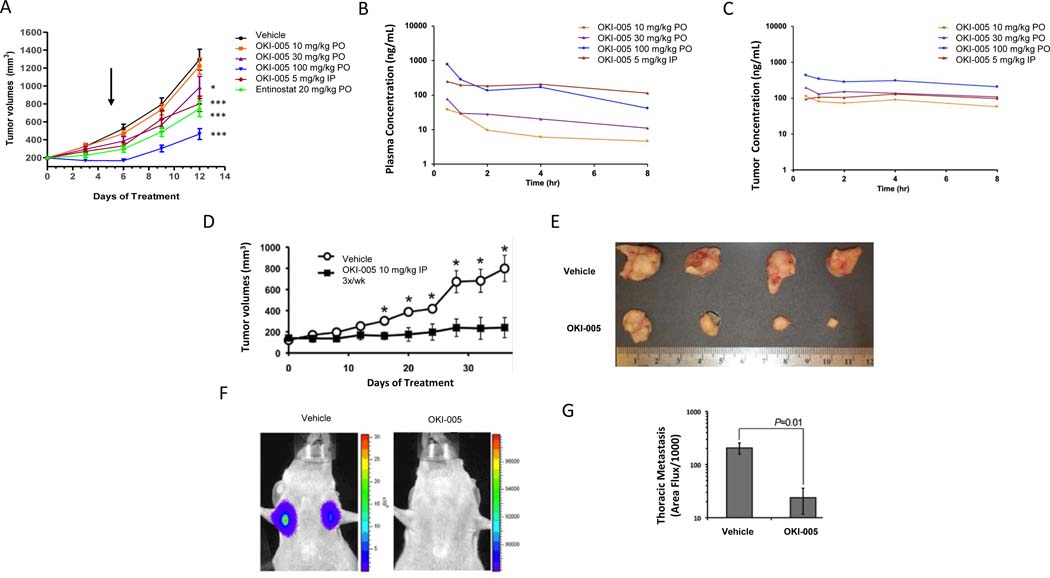
Figure 3.
Effect of OKI-005 in CRC and TNBC models and OKI-005 plasma and tumor pharmacokinetics. A) HCT116 (CRC) xenograft model in BALb/c nude mice treated with vehicle, OKI-005 (PO or IP) or etinostat × 12 days. Arrow indicates time of dose reduction in OKI-005 100 mg/kg PO group due to weight loss. * P < 0.05, *** P < 0.001 compared to vehicle group. B) Mean plasma concentration-time profiles of OKI-006 following a single dose of OKI-005 (PO or IP) in BALb/c nude mice. C) Mean tumor concentration-time profiles of OKI-006 following single dose of OKI-005 (PO or IP) in BALb/c nude mice. D) Luciferase-labeled MDA-MB-231 TNBC were engrafted onto the mammary fat pads of 6-week-old female BALB/c nude mice, which were left untreated (vehicle) or treated with OKI-005 IP (10 mg/kg, 3X/week IP). Data are the mean (± SEM) tumor volumes measured at the indicated time points after engraftment (*P < 0.05). E) MDA-MB-231 tumors excised at day 35 following treatment with either vehicle or OKI-005. F & G) Mice treated with OKI-005 as in panel E displayed significantly decreased pulmonary metastasis at day 35 post-engraftment. Data are expressed as the mean (± SEM). A t-test was used to compare groups.