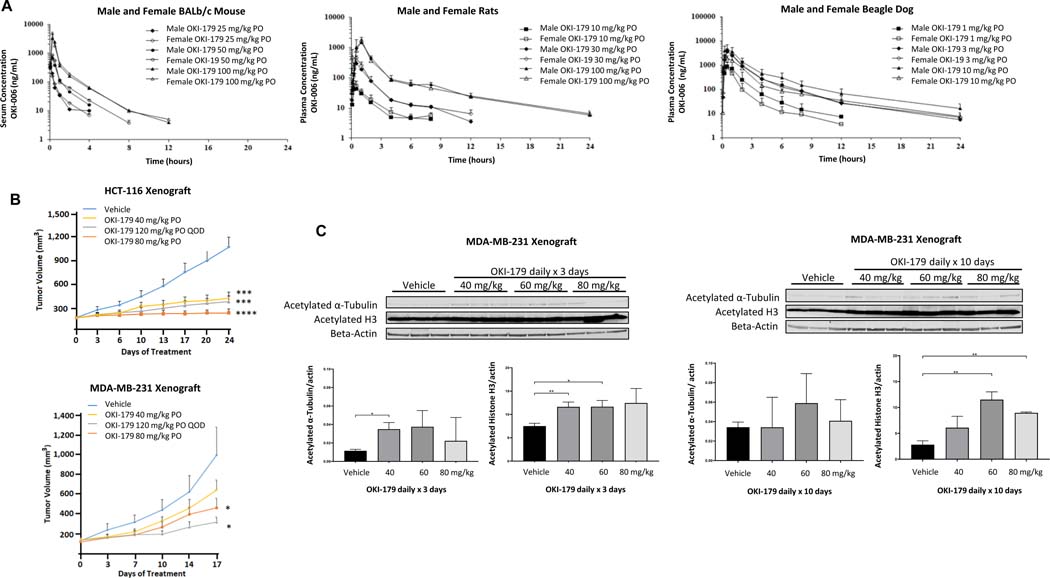
Figure 4.
Pharmacokinetic profile of OKI-179 and effects in vivo in CRC and TNBC xenograft models. A) Serum/plasma concentration-time profiles of OKI-006 following a single dose of OKI-179 PO in male and female BALB/c nude mice, rats and beagle dogs. B) Effect of OKI-179 in HCT116 and MDA-MB-231 xenograft models in BALB/c nude mice. Treatment groups were compared to vehicle control for end of treatment statistical analysis. C) Effect of OKI-179 treatment PO daily × 3 or 10 days in MDA-MB-231 xenograft model. Tumors were excised, protein extracted and expression of acetylated proteins assessed by immunoblotting. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001.