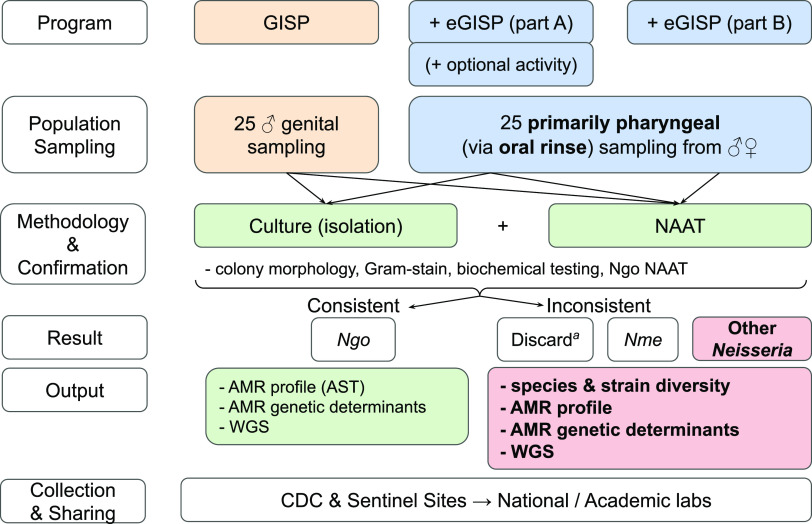
FIG 2.
Flowchart of the current protocol for the Gonococcal Isolate Surveillance Project (GISP, run by the CDC). For each clinical collection site, the current GISP (orange) and eGISP (blue) programs sample 25 individuals from genital and 25 from nongenital sites per month, respectively. Currently, all samples are submitted for morphological testing and nucleic acid amplification testing (NAAT) and retained if bacterial isolates are Gram-negative, diplococci, oxidase-positive, have a tan/transparent colony morphology on modified Thayer-Martin media, and NAAT-positive for N. gonorrhoeae (Ngo). aSentinel sites not participating in eGISP discard their non-Ngo samples. Retained colonies are then subjected to AMR profiling and WGS. We propose a minor modification to the eGISP program (bold and red), in which all pharyngeal samples collected via oral rinse-and-gargle (138) are conserved if they display features inconsistent with N. gonorrhoeae, yet consistent with other members of the Neisseria genus (Gram−, diplococci or rods, and oxidase+) (bold and red). We also suggest that pharyngeal samples are inoculated onto LBVT.SNR media, which is selective for commensal Neisseria. Subsequent development of a pipeline for complete analysis of each resultant non-N. gonorrhoeae strain (AMR profiling, WGS, etc.) will allow for detailed characterization of Neisseria species and strain diversity, allowing for a comprehensive evaluation of the Neisseria resistome and other comparative genomics analyses of interest.