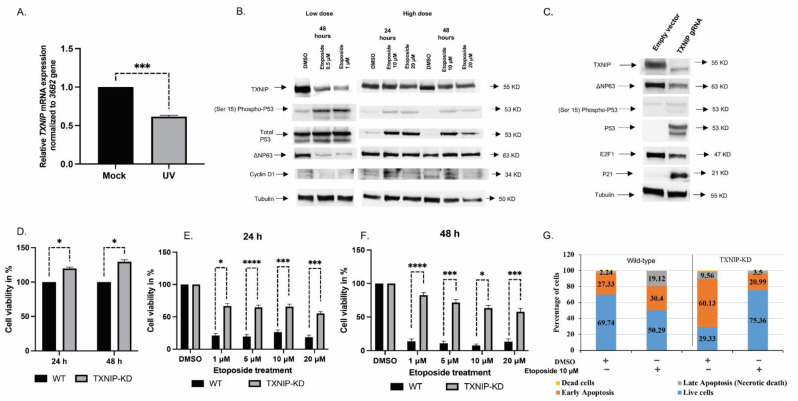
Figure 1.
Analysis of the role of TXNIP in the DNA damage response. (A) Primary normal fibroblast cells (3 individual cell lines) were irradiated with UV (50 mjoule/cm), or left untreated, and harvested at 6 h for RT-QPCR analysis. *** p < 0.001 vs. respective untreated cells. (B) HEK293t cells were stimulated with low (0.5 μM and 1 μM) or high (10 μM and 20 μM) doses of etoposide for 24 or 48 h. After separation by SDS-PAGE, protein levels of TXNIP, P53, phospho-P53 (phosphorylated at serine 15), ΔNP63 and Cyclin D1 were determined using immunoblotting. Tubulin served as a loading control. Results shown are means ± S.D. of two independent experiments with duplicate samples. (C) HEK293t cells were maintained in full medium and transfected with a CRISPR/Cas9 plasmid containing a gRNA for the TXNIP gene (or non-targeting gRNA). Cells were sorted based on the expression of GFP. At the end of the incubation period, cells were harvested and lysed as described in Section 2. Lysates (100 µg) were analyzed by Western blotting for TXNIP, P53, phospho-P53 (phosphorylated at serine 15), P21, Cyclin D1, E2F1 and ΔNP63. Levels of tubulin were measured as a loading control. (D) TXNIP-KD HEK293t and wild-type cells were seeded in 96-well plates (3000 cells/well; four replicates) for 24 or 48 h, after which cell proliferation was assessed by XTT assays. A value of 100% was given to the cell number of wild-type cells. The experiment was repeated three times with similar results. (E,F) TXNIP-KD HEK293t and wild-type cells were treated with increasing doses of etoposide for 24 (E) or 48 (F) h, after which cell viability was measured by XTT assays. A value of 100% was given to the number of cells of DMSO-treated cultures. Shown is a typical experiment repeated three times with similar results. * p-value ≤ 0.05; *** p-value ≤ 0.001; **** p-value ≤ 0.0001 (G) Etoposide-treated wild-type and TXNIP-KD HEK293t cells were analyzed by flow cytometry to distinguish between apoptotic and necrotic cell death. Annexin V FITC/PI staining was used to assess the mode of cell death.