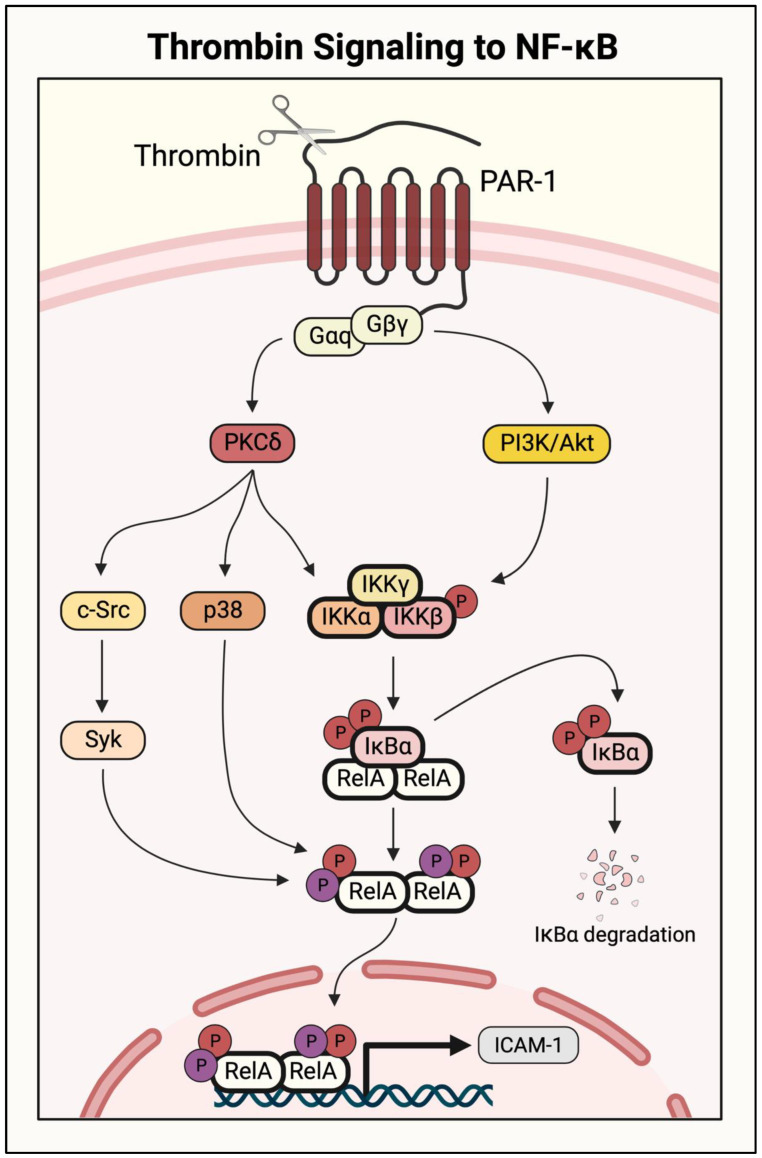
Figure 7.
Thrombin-activated NF-κB pathway in endothelial cells. Thrombin activates its receptor, the G-protein-coupled receptor (GPCR) protease-activated receptor (PAR-1), by cleaving the extracellular domain to stimulate signaling. The Gβγ subunit activates phosphatidylinositol-3 kinase (PI3K)/Akt, which phosphorylates the IKK complex to activate canonical NF-κB signaling and induce RelA/p65 homodimer translocation to the nucleus. Gαq phosphorylates protein kinase C delta (PKCδ), which activates NF-κB by dual mechanisms. It induces IKK complex phosphorylation to release RelA/p65 for its translocation and DNA binding in the nucleus. It also engages p38 and c-Src/spleen tyrosine kinase (Syk) to phosphorylate RelA/p65 and enhance its transcriptional activity. Whereas p38 causes serine phosphorylation (red), c-Src and Syk cause tyrosine phosphorylation (purple).