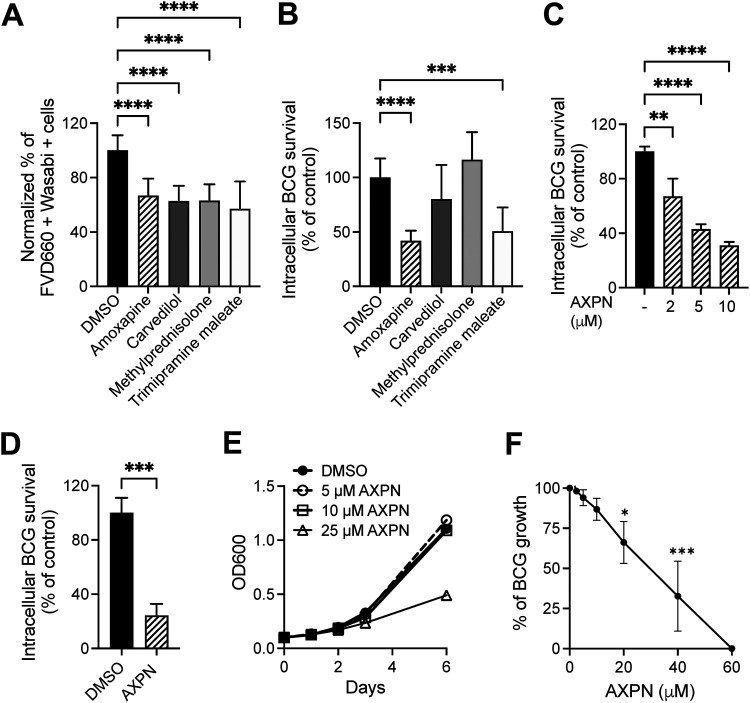
FIG 1.
FDA-approved drugs reduce Mycobacterium bovis BCG-induced cytotoxicity and inhibit intracellular BCG survival in macrophages. (A) RAW 264.7 cells were treated with drugs at 10 μM concentration after BCG infection for 3 h, and cytotoxicity was determined by Fixable Viability Dye eFluor 660 staining on day 1 postinfection. Data show the percentage of infected dead cells in drug-treated groups versus the DMSO-treated group. (B) Intracellular BCG survival was determined in RAW 264.7 cells with BCG infection and postinfection treatment with 10 μM drugs on day 2 postinfection. (C) RAW 264.7 cells were infected with BCG at a multiplicity of infection (MOI) of 10 for 3 h and then treated with Amoxapine at the indicated concentrations. Intracellular BCG survival was determined on day 2 postinfection. (D) THP1 cells were treated with 10 μM Amoxapine after 3 h of BCG infection, and intracellular BCG survival was enumerated on day 3 postinfection. (E) To determine the direct bactericidal activity of Amoxapine, BCG growth in 7H9+OADC liquid broth culture was determined by measuring optical density at 600 nm (OD600) at different time points with various concentrations of Amoxapine. (F) A resazurin microtiter assay was used to evaluate the sensitivity of BCG to Amoxapine treatment after 5 days of incubation. Data are shown as means ± standard deviations for three independent experiments. One-way analysis of variance (ANOVA) with Dunnett’s multiple-comparison test or Student’s t test was used to compare drug-treated groups to the control group. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.