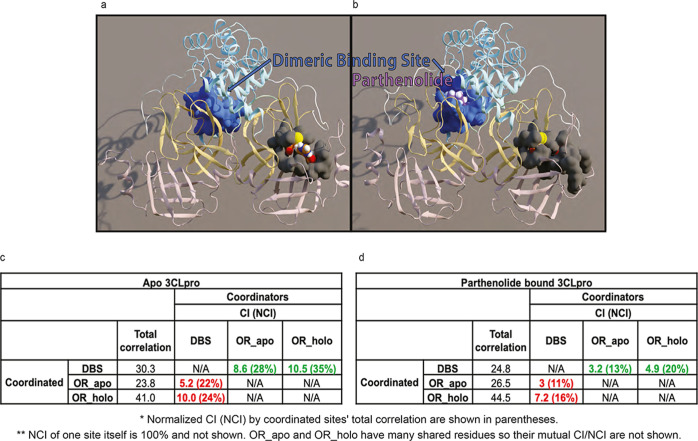
Figure 8.
PRT disrupts the allosteric communication between the DBS and the catalytic site of 3CLpro. (A) Apo structure, with the residues in the dimeric binding site shown in a blue surface and residues that occlude the catalytic dyad shown in a gray surface. (B) In the complex with PRT (in purple) bound in the DBS, the residue groups are shown in the same colors as in A. Note the dramatic reduction in accessibility to the catalytic dyad. (C) Values of the TC. CI and NCI (in parentheses) are shown for apo 3CLpro, calculated with the DBS as the coordinator and the occluding residues defined from the apo and holo trajectories (OR_apo/OR_holo) as coordinated (values highlighted in red). For comparison, values from the corresponding reversed calculation (DBS is coordinated; occluding residue groups are coordinating) are highlighted in green. (D) Results from the same calculations as in (C), from the PRT-bound 3CLpro trajectories.