Fig. 5.
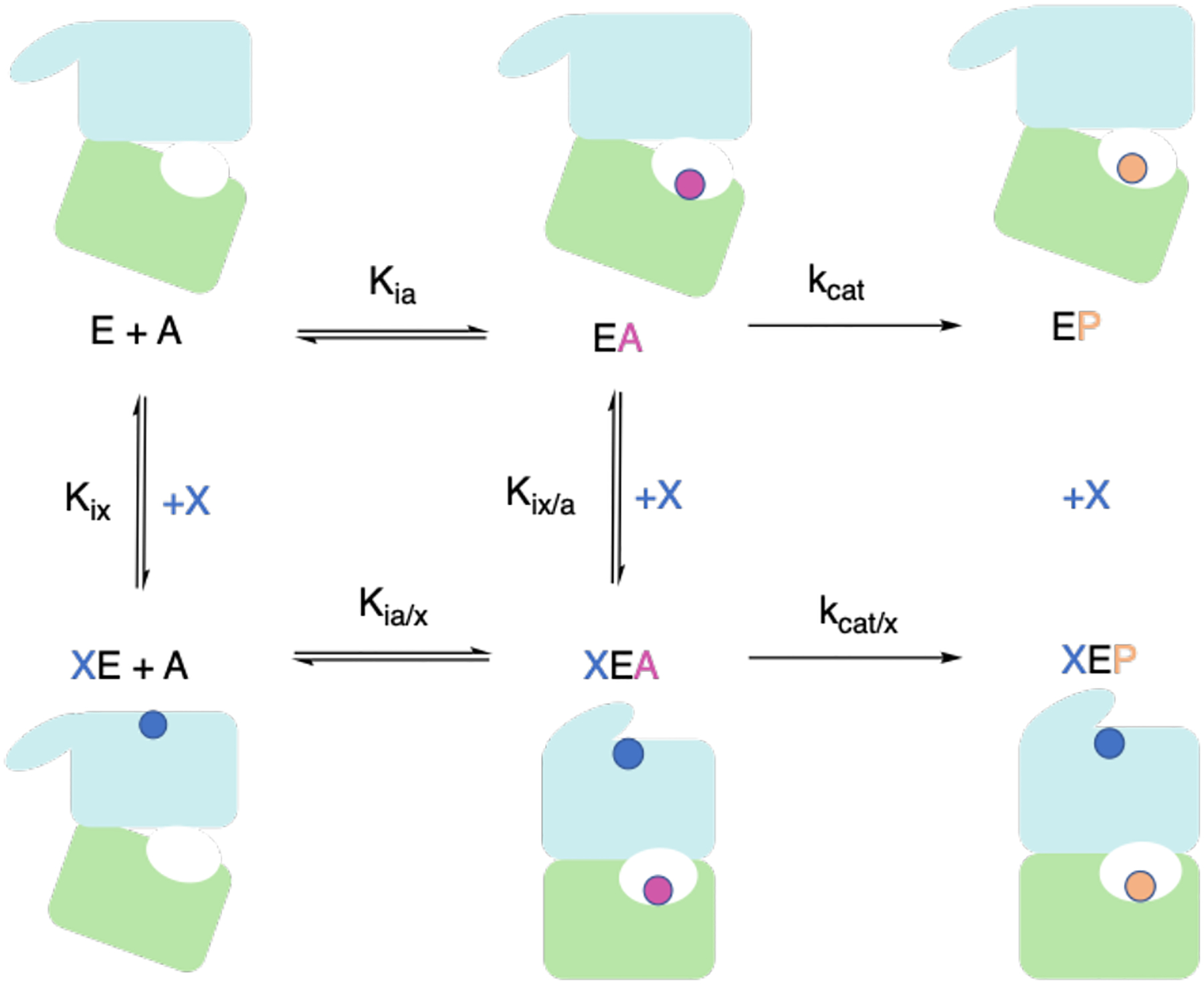
Complete thermodynamic cycle of an allosteric enzyme, E. The top row represents the enzyme in the presence of only substrate, A. The bottom row represents the enzyme in the presence of the substrate and allosteric effector, X. Binding of X modifies the activity of E by either altering the binding affinity of E for A, as in K-type allostery, or influencing the rate-determining step, as in V-type allostery. Nomenclature from Ref. [83] is used here for consistency.
