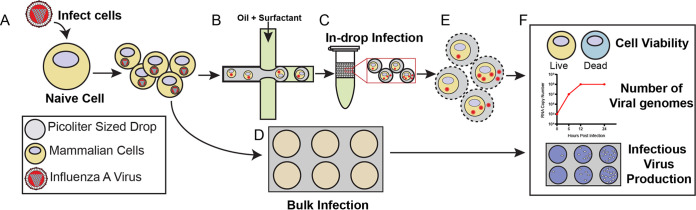
FIG 1.
Graphical workflow. (A) Cells were infected with IAV. (B) A suspension of infected cells was encapsulated into drops using a fluorinated oil continuous phase. (C) Drops were incubated for 24 h at 37°C and 5% CO2. (D) A portion of infected cells was replated onto standard tissue culture dishes to recapitulate a bulk infection and incubated for 24 h at 37°C and 5% CO2. (E) To analyze virus production, the drops were broken and pooled, and cells and/or viral supernatant were recovered. (F) Cells and virus from drop and bulk infections were analyzed using LIVE/DEAD staining to determine cell viability, RT-qPCR to determine viral genome copy number, and plaque assays to determine viral infectivity.