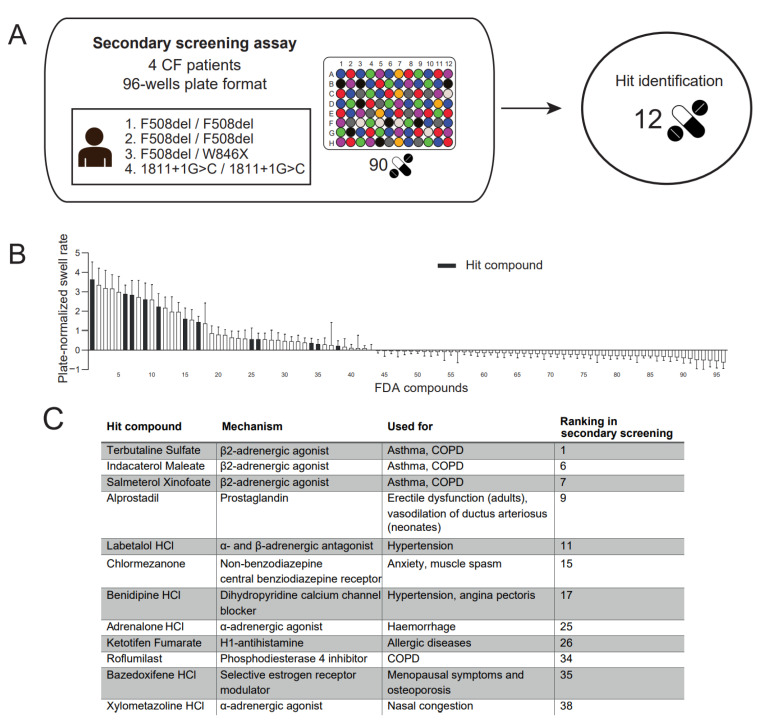
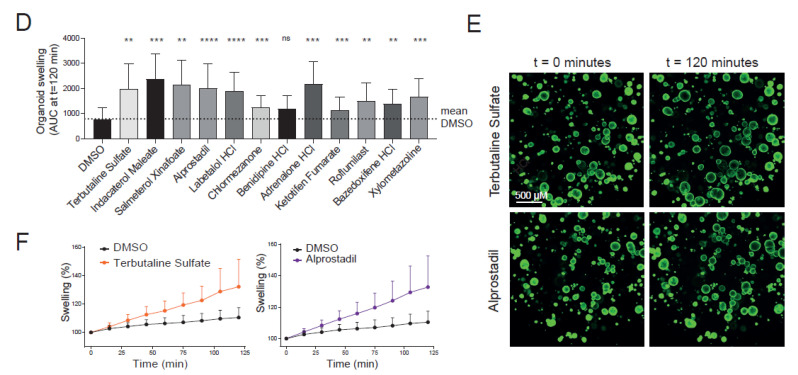
Figure 4.
Secondary screening assay and validation of FDA hit compounds in CFTR-null donors. (A) A total of 90 hit compounds identified in the primary screening assay were further validated in the conventional 96-well plate format with one compound per well; (B) the graph represents mean plate-normalized swell rates of four donors. Hit compounds were selected based on swell rate and working mechanism (n = 4 independent donors; F508del/F508del, F508del/F508del, F508del/W846X, 1811G+1>C/1811+1G>C, 3 replicates per donor); (C) overview of the 12 hit compounds with their working mechanism, disease application and ranking in the secondary screening assay; (D) the 12 hit compounds were further evaluated in an organoid swelling assay with CFTR-null nasal organoids (n = 3 independent donors: G542X/CFTRdele2.3(21kb), W1282X/1717-1G>A, R553X/R553X, n = 2–7 measurements per donor). The compounds are ranked based on their effect size in the secondary screening assay, shown in (B). The conventional image analysis was applied using fluorescent-labeled organoids. Organoid swelling is shown as AUC values from measurements of 120 min; (E) representative confocal images of CFTR-null (R553X/R553X) nasal organoids, stimulated with Terbutaline Sulfate or Alprostadil (both 3 µM) as example of two hit compounds at 0 and 120 min. (F) Quantification of CFTR-null (R553X/R553X) nasal organoid swelling after stimulation with Terbutaline Sulfate or Alprostadil (both 3 µM). Differences with baseline are analyzed using a one-way ANOVA with Dunnett’s post hoc test (D). ns = non-significant, ** p < 0.01, *** p < 0.001, **** p < 0.0001.