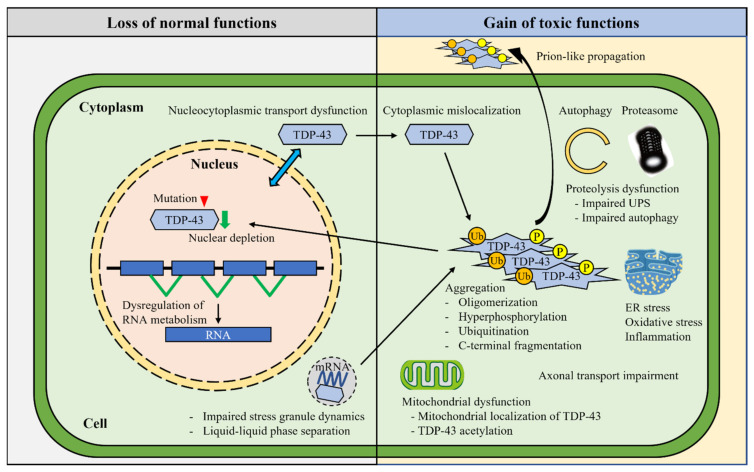
Figure 2.
Molecular mechanisms underlying TDP-43 pathogenesis. Various mechanisms have been identified in the TDP-43 pathogenesis. The impairments of TDP-43 autoregulation and nucleocytoplasmic shuttling result in the dysregulation of RNA metabolism and increased cytoplasmic TDP-43 mislocalization. TDP-43 mislocalization triggers various post-translational modifications, including hyperphosphorylation, ubiquitination, acetylation, and C-terminal fragmentation, which facilitate TDP-43 oligomerization and aggregation. The FALS-linked mutations enhance intrinsic aggregation. The nuclear depletion and cytoplasmic aggregation of TDP-43 induce multiple cytotoxic effects, such as aberrant stress granule dynamics, liquid–liquid phase separation, mitochondrial dysfunction, endoplasmic reticulum (ER) stress, impaired axonal transport, and proteolysis dysfunction. TDP-43 aggregates show prion-like cell-to-cell propagation, which may confer the disease progression.