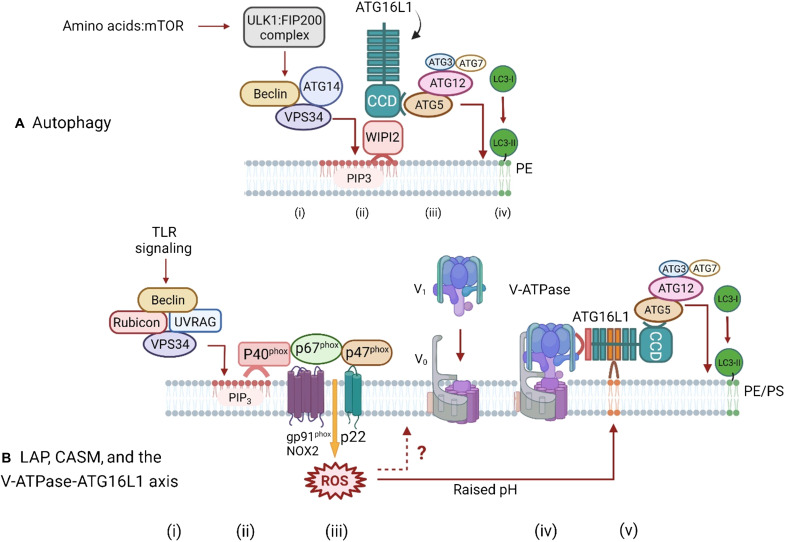
Fig. 2. Pathways for ATG16L1-mediated conjugation of LC3 to membranes during autophagy and LAP/CASM conjugation via the V-ATPase-ATG16L1 axis.
(A) Autophagy. Autophagy is activated in response to a fall in amino acids, which leads to inhibition of mammalian target of rapamycin (mTOR) and activation of the ULK1/FIP200 initiation complex (i) and downstream activation of the PI3K complex containing Beclin1, ATG14, and VPS34. The PI3K activity of VPS34 generates PIP3 in autophagosome membranes, which provide a platform for binding of WIPI2 (ii). WIPI2 binds to the coiled coil domain (CCD) of ATG16L1, leading to recruitment of the LC3 conjugation complex (ATG5-ATG12, ATG3, and ATG7). The conjugation reaction results in conversion of LC3I to LC3II and covalent binding of LC3 to PE in the autophagosome membrane (iii and iv). (B) LAP/CASM and the V-ATPase-ATG16L1 axis. LC3 conjugation in phagocytic cells is activated by TLR signaling through a complex containing Rubicon, Beclin1, UVRAG, VPS15, and VPS34 (i). TLR signaling activates VPS34 within the complex leading to generation of PIP3 in phagosome membranes (ii) to generate a binding site for p40phox that stabilizes the NADPH complex (NOX2, p47phox, p40phox, and p67phox). At the same time, binding of Rubicon to p22phox increases production of reactive oxygen species (ROS). Generation of ROS increases the pH in the lumen of the phagosome (iii) stimulating assembly of the Vo V1 subunits of Vo-V1 (iv). It is also possible that ROS can act directly on V-ATPase (?). V-ATPase binds to the WD domain of ATG16L1, leading to recruitment of the LC3 conjugation complex (ATG5-ATG12, ATG3, and ATG7) to the phagosome and conjugation of LC3 to phosphatidylserine (PS) and phosphatidylethanolamine (PE) in the phagosome membrane (v). A similar ROS-dependent pathway involving assembly of V-ATPase operates in nonphagocytic cells, but the precise components of the NADPH oxidase complex are unclear.