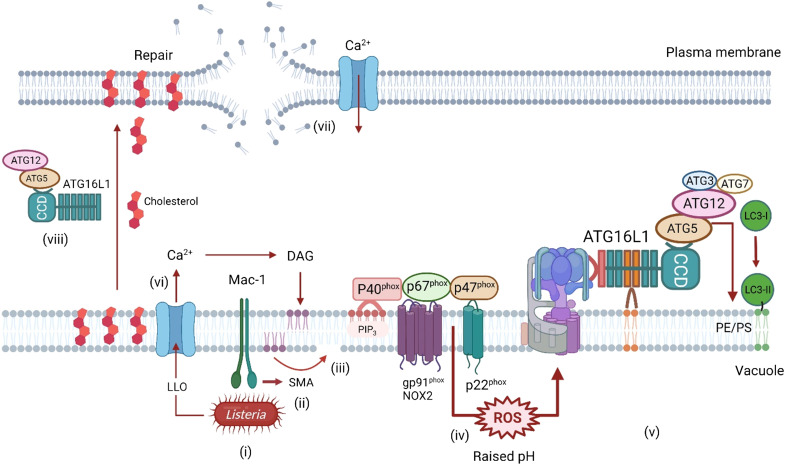
Fig. 7. Recruitment of LC3 to membranes during Listeria infection.
Binding of Listeria to Mac-1 in vacuoles in macrophages (i) activates sphingomyelinase (SMA), which removes the phosphocholine head group from sphingomyelin to generate ceramide (ii and iii). Ceramide-enriched microdomains facilitate assembly of the NADPH oxidase/NOX2 complex and enhance production of ROS (iv), which leads to assembly of V-ATPase and recruitment of ATG16L1:ATG5-ATG12 to conjugate LC3 to the vacuole membrane (v). LLO is a pore-forming toxin release by Listeria. Damage to the vacuole and subsequent entry of Ca2+ into the cytosol (vi) induce production of DAG, which activates the NADPH oxidase/NOX2 complex directly. Damage to the plasma membrane by LLO and entry of Ca2+ into the cytosol trigger a membrane repair pathway, which is dependent on the WD domain of ATG16L1 and ATG5-ATG12 (vii and viii). This may involve transport of cholesterol from vacuoles to the plasma membrane and lysosome-mediated membrane repair.