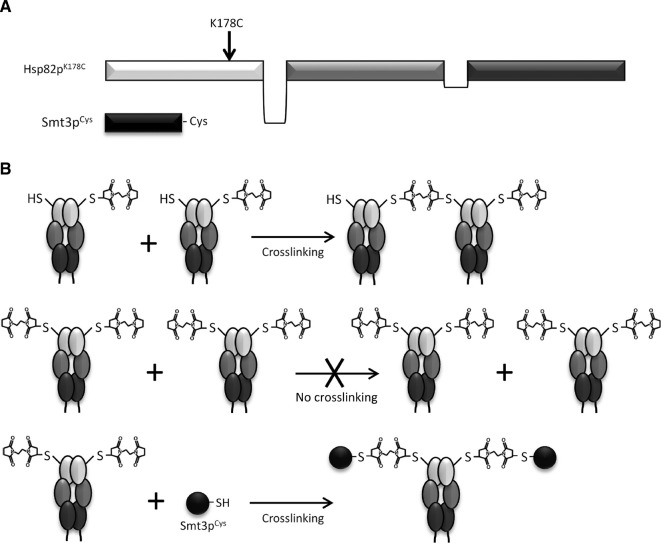
Figure 1:
Constructs and crosslinking strategy for the covalent attachment of Smt3pCys to Hsp82pK178C.
(A) Full-length Hsp82p construct harboring a cysteine in place of lysine at residue 178, and mature form of Smt3p harboring a cysteine downstream of the diglycine motif. (B) Using the homo-bifunctional maleimide crosslinker, BMOE, singly derivatized Hsp82pK178C will crosslink to itself to form Hsp82pK178C-Hsp82pK178C dimers. However, if derivatized with a vast excess of BMOE, every molecule of Hsp82pK178C will be conjugated to BMOE thereby preventing the formation of Hsp82pK178C-Hsp82pK178C crosslinked dimers. When all molecules of Hsp82pK178C are derivatized then near-complete crosslinking to Smt3pCys can be achieved.