Figure 5:
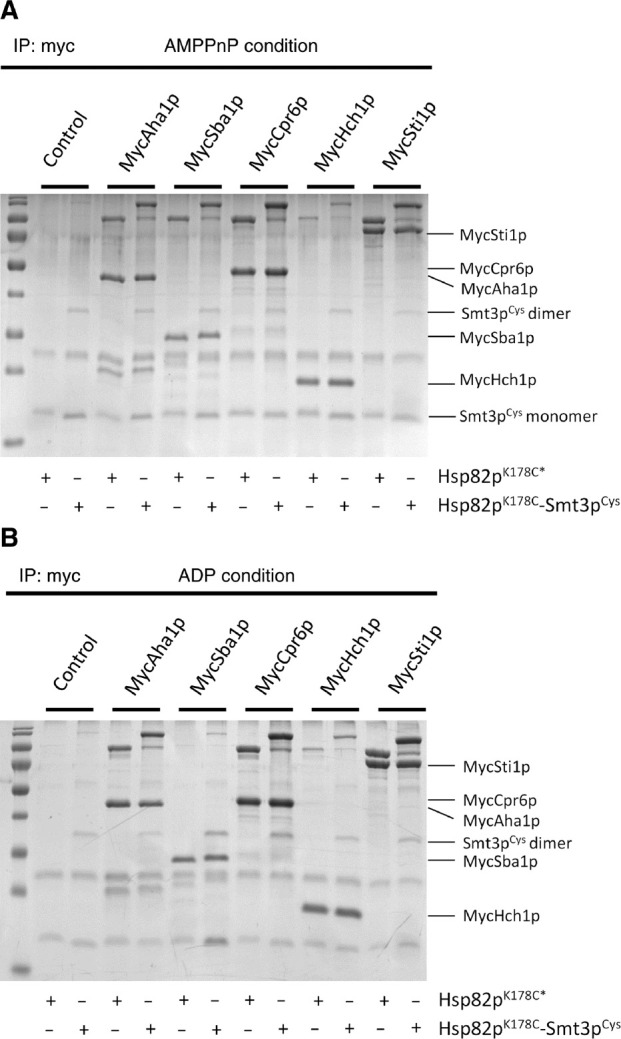
Co-chaperones interact with non-SUMOylated and SUMOylated-Hsp82p equally.
Myc-tagged Aha1p, Sba1p, Cpr6p, Hch1p and Sti1p immunoprecipitated non-SUMOylated and SUMOylated Hsp82pK178C to the same extent in both AMPPnP (A) and ADP (B) conditions. Control lanes show the amount of Hsp82pK178C recovered with beads alone, indicating non-specific binding of SUMOylated Hsp82p. Reactions contained 5 μm derivatized Hsp82p (Hsp82pK178C*), in the presence or absence of Smt3pCys, which was then quenched with 10 mm DTT for 15 min prior to mixing with 5 μm of Myc-tagged co-chaperones. These 50 μl reactions were incubated for an hour at room temperature. Ten microliters of Ultralink Protein G beads coupled to anti-Myc monoclonal antibodies were added to the 50 μl reactions and then incubated on a rotator at room temperature for 90 min. Beads were then pelleted, washed once in 250 μl of binding buffer, and resuspended in 50 μl SDS sample buffer. Ten microliters of each reaction were run on 12% SDS-PAGE gel and complexes were analyzed by Coomassie blue staining. IP experiments were conducted 3 times (n=3) and representative gels showing the individual reactions are shown in (A) and (B).
