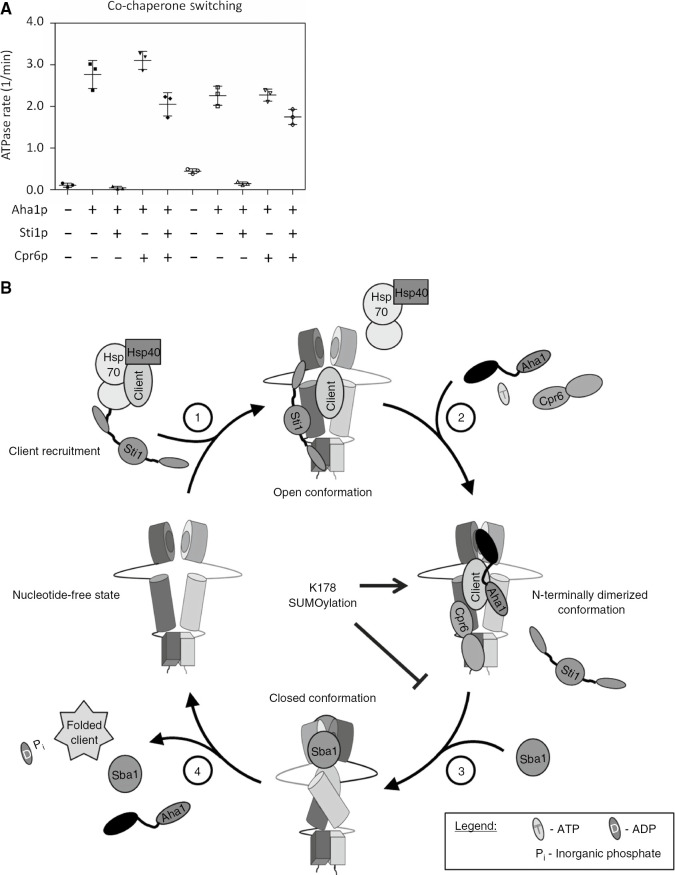
Figure 6:
The cooperative displacement of Sti1p from SUMOylated Hsp82p and a model for SUMO regulation of the ATPase cycle.
(A) Effective displacement of Sti1p by Cpr6p and Aha1p restore ATPase activity of SUMOylated Hsp82pK178C to a similar degree as non-SUMOylated Hsp82pK178C. Reactions contained 1 μm Hsp82pK178C (black) or Hsp82pK178C-Smt3pCys (gray) and 4 μm Sti1p, Cpr6p, and Aha1p where indicated. Each scatter plot shows the average and standard deviation from three triplicate experiments (n=3). (B) A model of the role SUMOylation has on its ATPase cycle of Hsp90. SUMOylation of yeast Hsp90 at K178 does not interfere with Sti1p displacement, but results in a stable recruitment of Aha1p which hinders Sba1p inhibition.