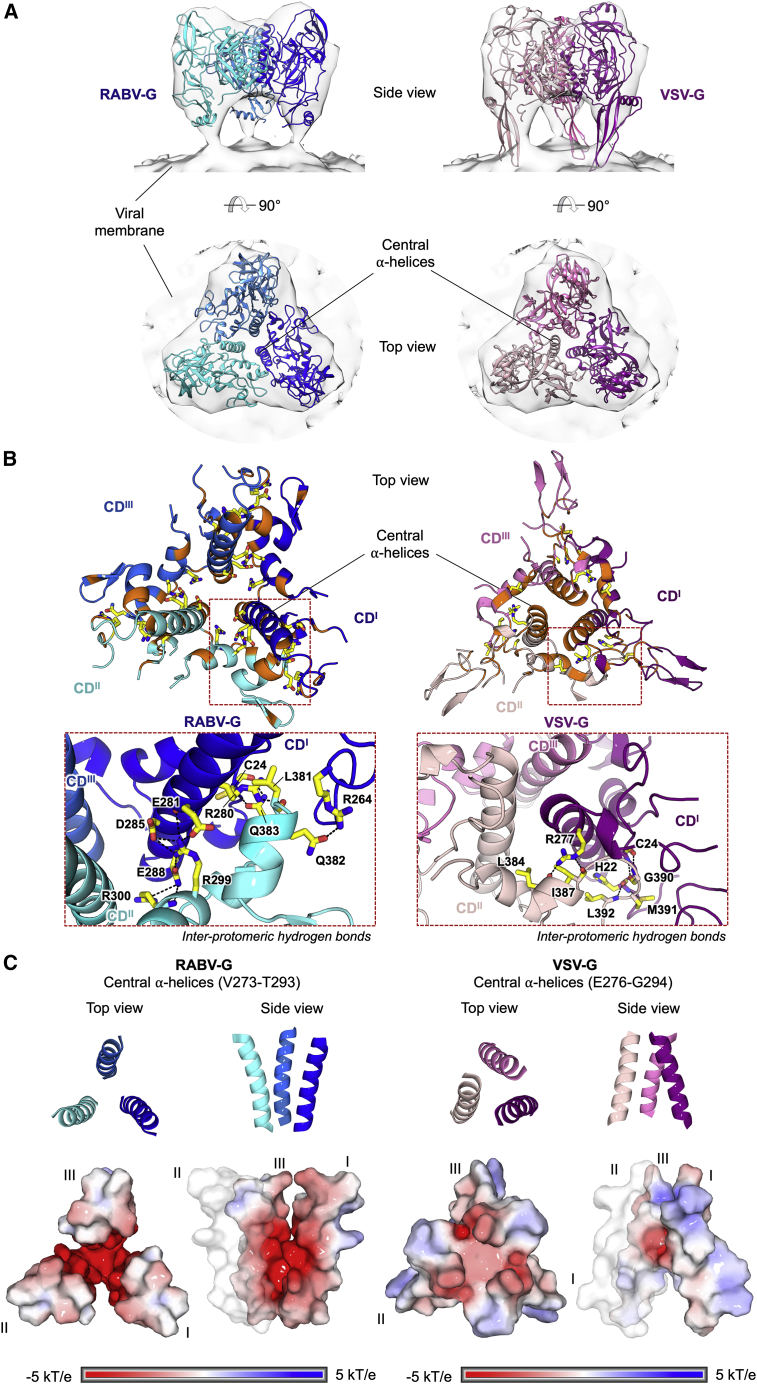
Figure 3.
Structural comparison of pre-fusion RABV-G and VSV-G reveals contrasting modes of inter-protomeric interactions at the trimerization core
(A) Fitting of pre-fusion RABV-G (blue) and VSV-G (pink; PDB: 5I2S) into a subtomographic average map of pre-fusion VSV-G (semi-transparent gray; EMD-9331) (Si et al., 2018). RABV-G and VSV-G structures are shown in cartoon representation and colored in different shades of blue and pink, respectively. Maps are shown as transparent surfaces.
(B) Zoom-in views of the G trimerization core involved in inter-protomeric interactions. Residues involved in hydrogen bonding (black dashed lines) are shown as sticks, with the carbon, nitrogen, oxygen, and sulfur constituents colored yellow, blue, red, and dark yellow, respectively. Residues involved in non-polar interactions are colored orange. The zoom-in panels of the CD α-helices shows the inter-protomeric hydrogen bonds. This analysis demonstrates that the trimerization core of RABV-G is largely mediated by polar interactions between charged residues, including a network of hydrogen bonds formed by negatively charged Glu281, Asp285, and Glu288 on one protomer and positively charged Arg299 and Arg300 on the adjacent protomer. Hydrophobic interactions are formed at the periphery and bottom of the central α-helices. In contrast, VSV-G displays the reverse pattern of inter-protomeric interactions, where the core is largely maintained by hydrophobic interactions with hydrogen bonds formed at the periphery.
(C) Electrostatic potential of the central α-helices. The central α-helices of RABV-G (left) and VSV-G (right) are shown as cartoons (top) and surfaces (bottom). The surfaces are colored according to the electrostatic potential in the range of ±5 kT/e, as calculated by adaptive Poisson-Boltzmann solver (APBS). Both RABV-G and VSV-G display negatively charged trimerization cores. This characteristic is especially prominent in RABV-G, where the carboxyl groups of Glu274, Glu275, Glu281, Glu282, Asp285, and Glu288 side chains line the central α-helices.