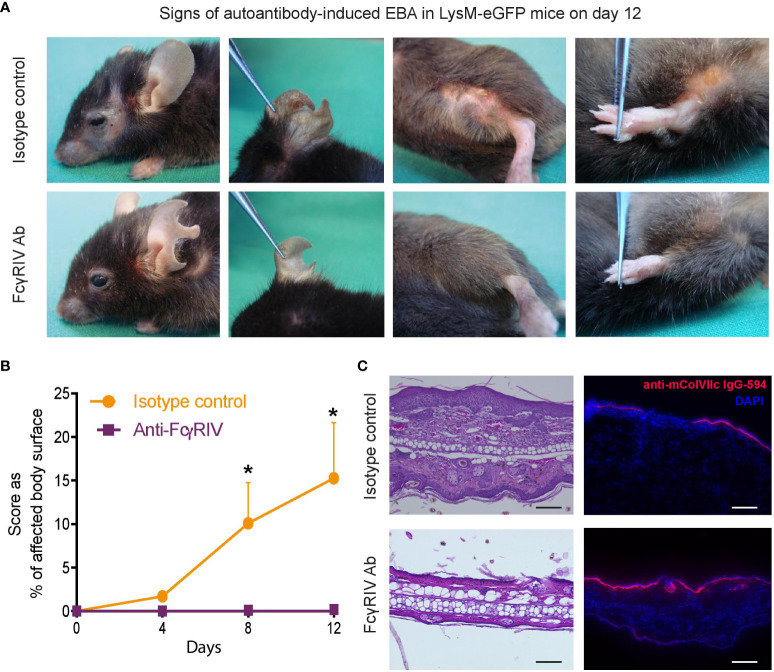
Figure 1.
Blocking the FcγRIV protects from induction of clinical disease manifestation in experimental EBA. Experimental EBA was induced by autoantibody transfer of DyLight594-labeled, anti-collagenVII IgG into LysM-eGFP mice treated with the function-blocking FcγRIV monoclonal antibody 9E9 (FcγRIV Ab) or IgG1κ isotype control. (A) Representative clinical pictures of mice on day 12. (B) A graph which displaying the clinical disease severity, expressed as percentage of body surface area covered by EBA skin lesions, on days 4, 8 and 12. Data is presented as mean and SD (n = 3) and analyzed using unpaired t-test: day 8, p < 0.0204; day 12, p < 0.0146. (C) HE staining of skin biopsies (ears) treated with anti-FcγRIV Ab or isotype control showed a pronounced dermal leukocyte infiltration, epidermal thickening and subepidermal blistering in isotype treated mice, while mice injected with 9E9 were devoid of histological features of EBA. Fluorescence imaging demonstrating anti-mColVIIc IgG-594 binding along the dermal-epidermal junction in both subgroups. Samples were obtained on day 12. Scale bar corresponds to100 µm. *p < 0.05.