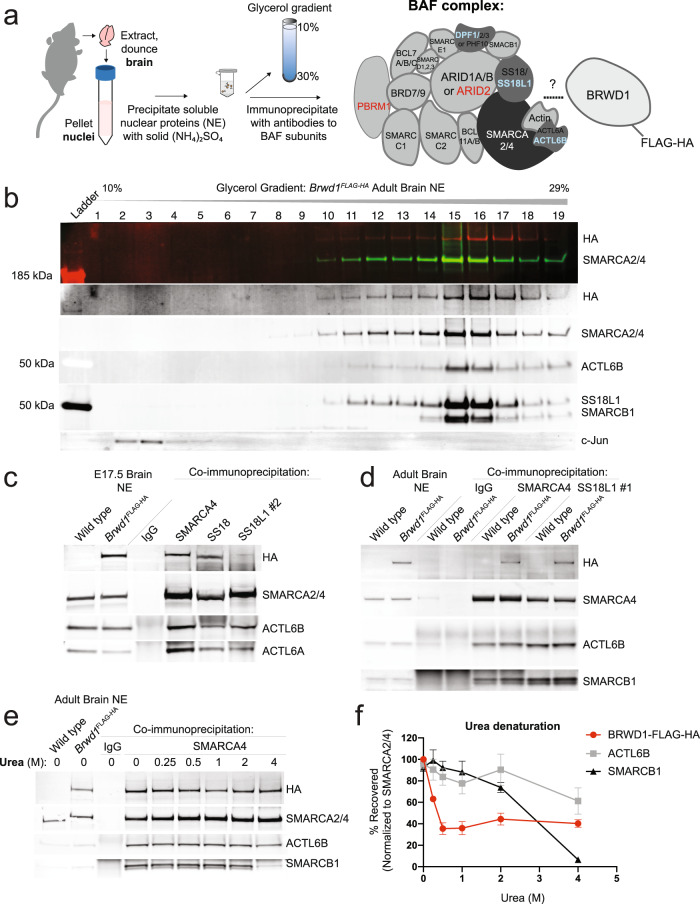
Fig. 3. BRWD1 tightly associates with the BAF complex in euploid brain.
a Schematic of mouse brain soluble nuclear protein extract (NE) preparation, density sedimentation of nuclear proteins over a 10–30% glycerol gradient, and immunoprecipitation of BAF chromatin remodeling complexes. Blue lettering indicates neuronal-specific BAF subunits. Red lettering indicates PBAF-specific subunits. b Density sedimentation of adult Brwd1FLAG-HA brain NE over a 10–30% glycerol gradient indicates that BRWD1 predominantly associates with large protein complexes. Subunits of BAF and AP-1 complexes serve as molecular weight markers: SMARCA2/4 antibody indicates all BAF complexes including non-canonical GBAF (~1 MDa)75, canonical BAF (~2 MDa) and Polybromo-containing BAF (PBAF, ~3 MDa); ACTL6B and SS18L1 indicate neuronal-specific BAF complexes; c-Jun indicates AP-1 (160–440 kDa). HA signal at the expected molecular weight of BRWD1-FLAG-HA (~260 kDa) is observed in fractions containing the BAF complex. c Endogenous BRWD1-FLAG-HA interacts with BAF complexes in embryonic brain. BAF complexes were immunoprecipitated from Brwd1FLAG-HA brain NE with antibodies against the BAF core ATPase SMARCA4, the neural progenitor subunit SS18, the neuronal subunit SS18L1 or IgG as a control. Endogenous BRWD1-FLAG-HA robustly co-immunoprecipitated with SMARCA4 and the neural progenitor subunit SS18, but less so with the neuronal subunit SS18L1 from E17.5 brain. d BAF complexes purified from adult Brwd1FLAG-HA brain NE with antibodies against SMARCA4 or the neuronal subunit SS18L1 co-immunoprecipitate BRWD1-FLAG-HA. e The stability of the BAF:BRWD1-FLAG-HA interaction was challenged with increasing concentrations (0.25-4 M) of the denaturing agent, urea. A fraction of BRWD1 remained bound to BAF in up to 4 M urea, surpassing the stability of the dedicated BAF subunit, SMARCB1. f Quantification of urea denaturation experiments, as shown in e, with the amount of bound protein normalized to the amount of immunoprecipitated SMARCA4 (n = 3 experiments). Source data are provided as a source data file. See Supplementary Fig. 15 for uncropped blots with MW markers.