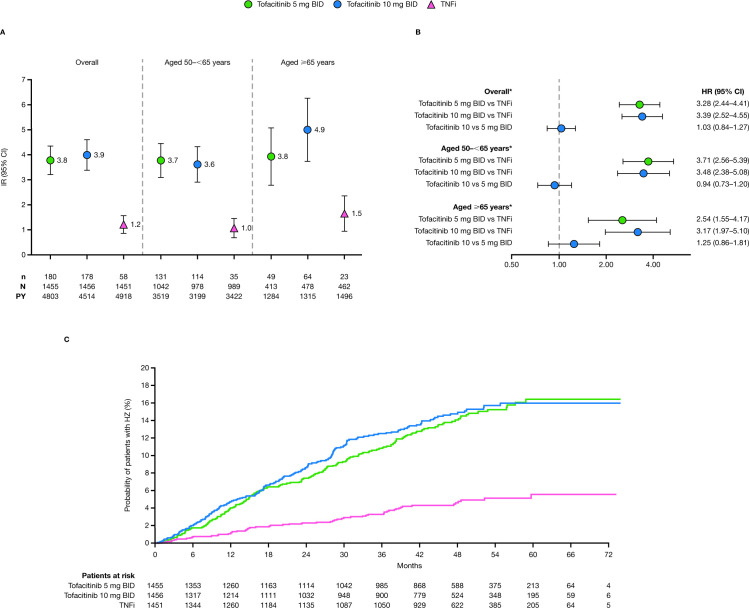
Figure 4.
(A) IRs (patients with first events/100 PY; 95% CIs) and (B) HRs (95% CIs) for all HZ (non-serious/serious), overall and stratified by age; and (C) cumulative probabilities of experiencing a first HZ (non-serious/serious) event (Kaplan-Meier method), in ORAL Surveillance. HRs are shown on a logarithmic scale. For patients randomised to the tofacitinib 10 mg two times per day group who had their dose of tofacitinib reduced to 5 mg two times per day, the data collected after patients were switched to tofacitinib 5 mg two times per day were counted in the tofacitinib 10 mg two times per day group. All HZ events (non-serious/serious) include HZ adjudicated as opportunistic infections and non-adjudicated HZ events from the clinical database. *HRs based on a multivariable Cox proportional hazard model for pairwise treatment comparisons with treatment, age, region, smoking and baseline corticosteroid use as covariates. BID, two times per day; HR, hazard ratio; HZ, herpes zoster; IR, incidence rate; N, number of evaluable patients; n, number of patients with events; PY, patient-years; TNFi, tumour necrosis factor inhibitors.