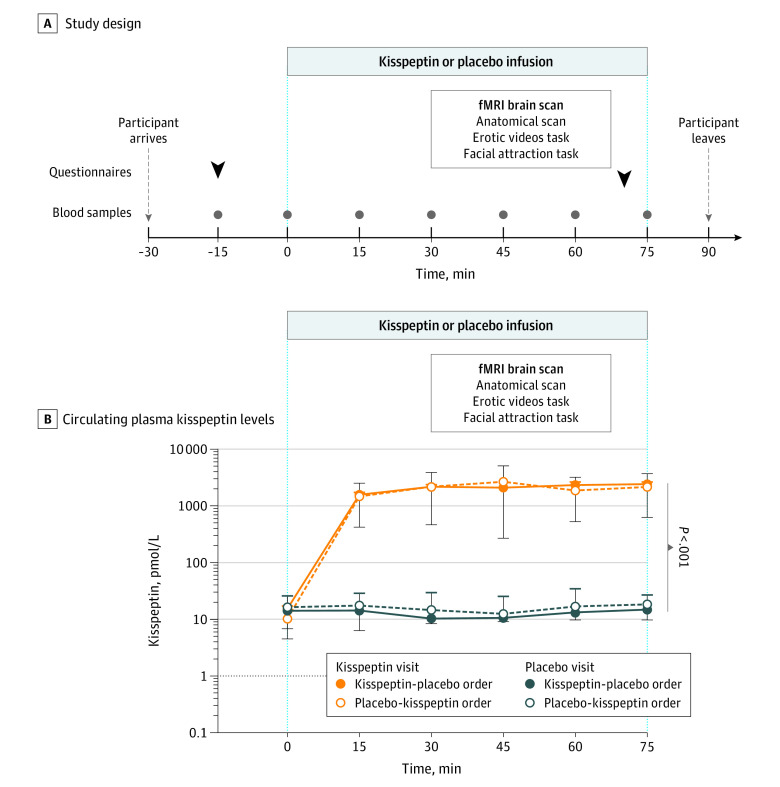
Figure 2. Experimental Protocol and Effects of Kisspeptin Administration on Circulating Kisspeptin Levels.
Participants attended 2 double-masked study visits at least 1 month apart (to allow full washout) in days 1 to 7 of the menstrual cycle: 1 for intravenous infusion of kisspeptin (1 nmol/kg/h) and 1 for intravenous infusion of equivalent volume of placebo for 75 minutes, in random order. In panel A, arrows indicate time points at which participants completed the following baseline and post-scan questionnaires: the Sexual Arousal and Desire Inventory, the State-Trait Anxiety Inventory Form Y-1 and the d2 Test of Attention. Participants underwent a functional MRI (fMRI) brain scan and the following types of scan and tasks were administered: anatomical (to evaluate any structural abnormality and for subsequent anatomical location), erotic videos task (watching 10 20-second short erotic videos with 10 20-second exercise control videos) and a facial attraction task (male and female faces of medium and high attractiveness). Dots indicate time points at which blood samples were taken for reproductive hormone levels. In panel B, kisspeptin administration resulted in increased circulating plasma kisspeptin levels.