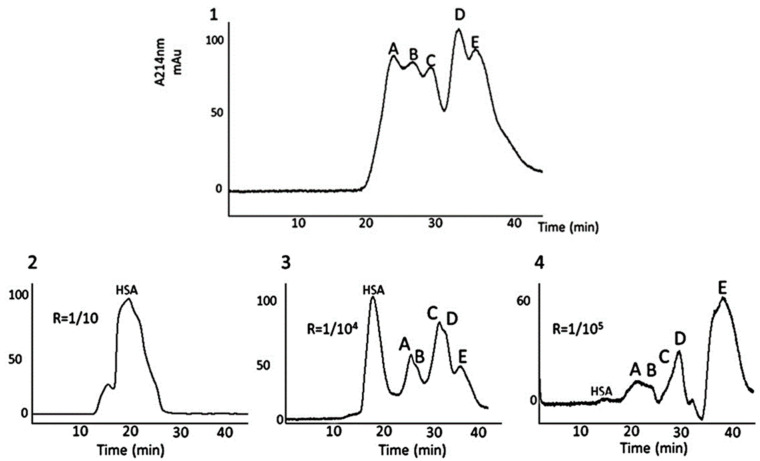
Figure 2.
Gel filtration HPLC of purified and oxidized VS-I samples to evaluate the impact of different concentrations of therapeutic human serum albumin (HSA) on multimers of VS-I. For the four graphs, the X-axis corresponds to the elution time (expressed in min), which is linearly related to the molecular weight of the components included in the peaks. The Y-axis corresponds to absorbance expressed in milliUnits of absorbance. Whether in vitro or in vivo, the oxidation of VS-I leads to multimerization, as shown on the first chromatogram (upper graph, numbered 1). The monomeric form of the peptide corresponds to peak E, while peaks A-D correspond to VS-I multimers. When adding fresh, non-oxidized therapeutic HSA at a molar albumin/VS-I ratio (R) from 1/10 to 1/105 (as shown on chromatograms 2–4), the release of the monomeric VS-I increases (see changes in the amplitude of peak E). This counterintuitive result explains the possible release of monomers of VS-I with the restoration of its anti-microbial properties [15].