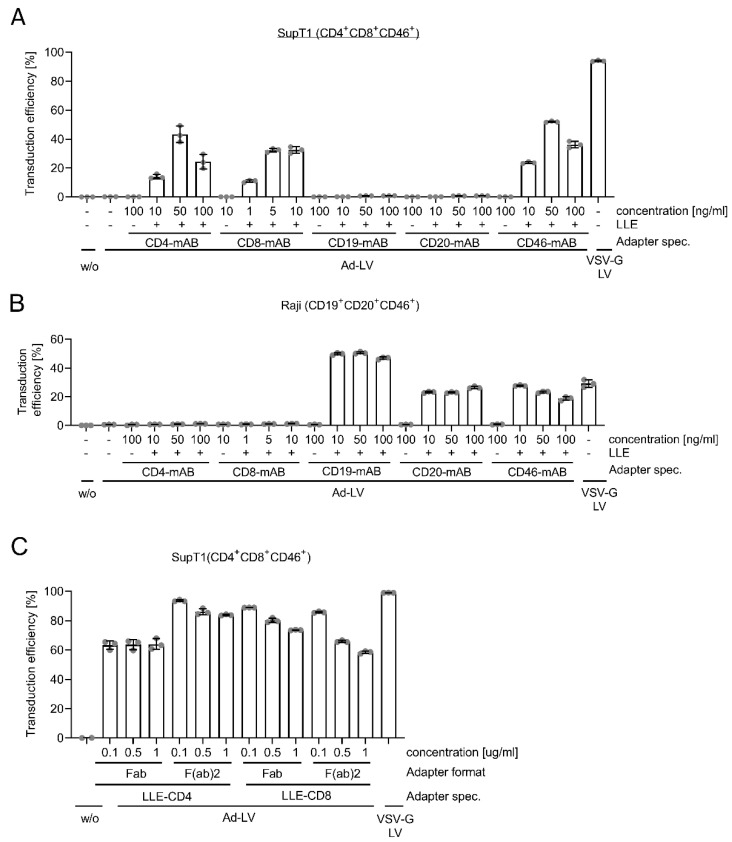
Figure 3.
Flexibility of Ad-LV towards adapter specificity and format. The flexibility of the adapter system was evaluated by transduction of cell lines using different adapter specificities. SupT1 (CD4 + CD8 + CD46+) (A) and Raji cells (CD19 + CD20 + CD46+) (B) were left untreated (w/o) or were transduced with GFP-encoding Ad-LV with 0.2 TU/cell using LLE-CD4 (clone: MT-466), LLE-CD8 (clone: BW135/80), LLE-CD19 (clone: LT19), LLE-CD20 (clone: LT20), and LLE-CD46 (clone: REA312) mABs (+) in concentrations ranging from 1 to 1000 ng/mL. Non-biotinylated mABs (-) used in the highest concentration and transduction in the absence of any adapter (-) were used to confirm specificity. VSV-G LV was used as reference. Transduction efficiency was analyzed 3 days (Raji) or 4 days (SupT1) post transduction via quantification of GFP-positive cells using flow cytometry. (C) Transduction efficiency of SupT1 comparing fab and f(ab)2 adapter formats of the same LLE-CD4 or LLE-CD8 clone with adapter concentrations ranging from 0.1 to 1 μg/mL using a GFP-encoding Ad-LV (0.1 TU/cell). VSV-G LV was used as reference. Data are represented as mean ± SD of 3 technical replicates.