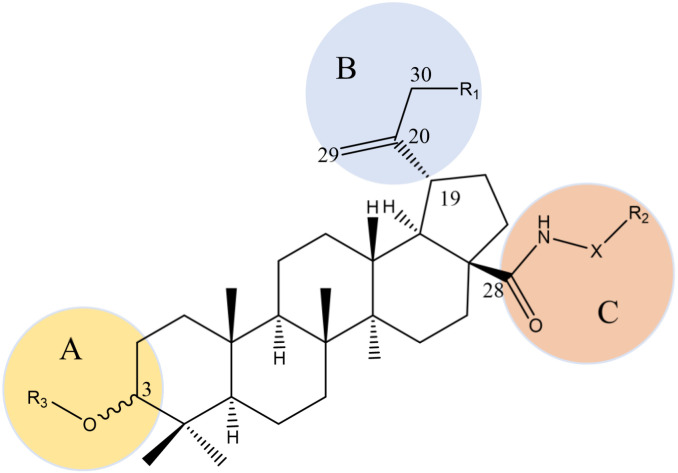
Figure 5.
Structure–activity relationships of BA derivatives against HIV. A: 1. Activity disappears after introduction of fluorine at the C-2 position, and the proton at the C-2 position may play a key role in anti-HIV activity. 2. 3-β configuration is better than 3-α configuration. 3. The position of the dimethyl substitution on the side chain greatly influences activity. 4.The activity of the C-3 ester group is replaced by the bioisostere amide. B: 1. The isopropenyl group at position C-19 may not be an active pharmacophore, but proper modification can improve the water solubility of the drug, and thereby improving the pharmacokinetic properties of the drug. 2. R1 as a hydrogen bond donor is unfavorable to the activity. 3. Changing the C-30 allyl group has no obvious effect on drug activity. C: 1. C-3 acylation can inhibit HIV maturation and C-28 amidation can prevent HIV from integrating into cell membranes. 2. The atomic length of the side chain at position C-28 is extremely significant for improving the antiviral efficacy, and the side chain structure containing amide groups is very important for activity. 3. The free hydroxyl group of R3 and the free carboxyl group at the end of R2 are necessary for anti-HIV activity. 4. When X is a secondary amine ring such as piperidine, it can significantly improve metabolic stability.