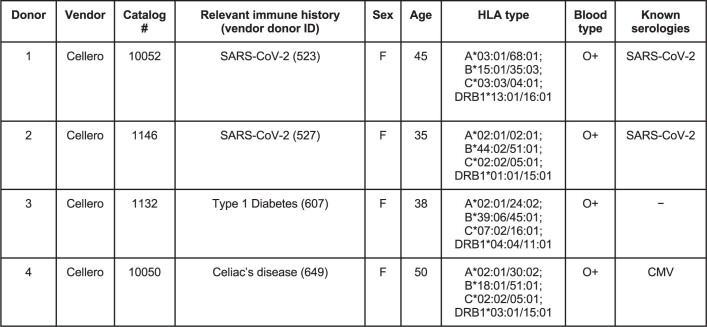
Extended Data Table 1.
Donor information
All donors were appropriately consented for genomic data use and release under protocols reviewed by independent IRB boards consulted by the vendor. Samples from the donors were tested by the vendor and confirmed to be both seronegative and not detectably infected with HIV-1, HIV-2, hepatitis B, hepatitis C, or HTLV-1. HLA typing, serology, and blood typing were also performed by the vendor. Donor 523 clinical timeline. Donor 523 tested positive for COVID-19 via RT-PCR of nasopharyngeal swabs on day 0 and was hospitalized from day −5 to day 0. She tested negative for COVID-19 at day 18, and had a plaque reduction neutralization test titer of 1:>2560 at day 44. She donated plasma and cells on day 65. Donor 527 clinical timeline. Donor 527 tested positive for COVID-19 via RT-PCR of nasopharyngeal swabs on day 0 and was not hospitalized. She tested negative for COVID-19 at day 15, and had a plaque reduction neutralization test titer of 1:20 at day 57. She donated plasma and cells on day 75.