Abstract
Caregivers who are higher in dispositional empathy tend to have children with better developmental outcomes; however, few studies have considered the role of child-directed (i.e., “parental”) empathy, which may be relevant for the caregiver–child relationship. We hypothesized that mothers’ parental empathy during their child’s infancy will be a stronger predictor of their child’s social-emotional functioning as a toddler than will mothers’ dispositional empathy. We further explored whether parental and dispositional empathy have shared or distinct patterns of neural activation during a social-cognitive movie-watching task. In 118 mother–infant dyads, greater parental empathy assessed when infants were 6 months old was associated with more social-emotional competencies and fewer problems in the children one year later, even after adjusting for dispositional empathy. In contrast, dispositional empathy was not associated with child functioning when controlling for parental empathy. In a subset of 20 mothers, insula activation was positively associated with specific facets of both dispositional and parental empathy, whereas right temporoparietal junction activation was associated only with parental empathy. Thus, dispositional and parental empathy appear to be dissociable by both brain and behavioral metrics. Parental empathy may be a viable target for interventions, especially for toddlers at risk for developing social-emotional difficulties.
Introduction
Empathy is multifaceted, comprising affective (i.e., sharing or resonating with others’ experiences), cognitive (i.e., understanding others’ experiences), and motivational (i.e., wanting to improve others’ states through one’s own actions) processes (Davis, 1983; Zaki, 2014). In addition, these aspects of empathy may be dispositional (i.e., directed to others in general) or to specific individuals (Davis, 1996). Parental empathy is empathy directed specifically toward one’s child (Stern, Borelli, & Smiley, 2014). Precisely how parental empathy differs from dispositional empathy in its associations with child development, however, is unknown. Given that parents are often children’s primary caregivers and the main source of social experience in the first years of life, the level of empathy in this relationship may have significant implications for children’s development. Specifically, parental empathy may facilitate parents’ attention to and recognition of their child’s needs and desires, potentially leading to engagement in higher quality caregiving behaviors that are linked to better social and emotional functioning in children (e.g., Pastorelli et al., 2016; Zhou, Eisenberg, & Fabes, 2002).
Multiple lines of research have found that parental empathy has positive implications for child development. For example, Oppenheim et al. (2001, 2002) found that greater parental cognitive empathy in mothers when their children were 4.5 years of age—which they called “empathic understanding” or “insightfulness”—was associated with more secure attachments in early life. Other research has demonstrated that mothers’ parental empathy and child-focused reflective functioning—understanding behaviors in terms of the actor’s mental states—were associated with children’s attachment security (Borelli et al., 2021a). Further evidence also suggests that mothers’ parental reflective functioning buffered the association between children’s observed distress and coping during a challenging behavioral task (Borelli et al., 2021b), underscoring the potential role of parental cognitive empathy in supporting children’s emotion regulation. Finally, Leerkes (2010) reported that mothers who found infant crying to be more aversive were less likely to respond sensitively to the infant’s needs, suggesting that caregivers’ emotion regulation difficulties prevent the modeling and training of children’s strategies for coping with difficult emotions in a way that facilitates prosocial engagement.
Miller et al. (2015) found that higher levels of parental empathy—which they called “compassionate love” for their children—was protective against harsh (self-reported) parenting and associated with greater (observed) warmth during a low-challenge mother-child task. Separate work validating a scale specific to parental cognitive and affective empathy found that greater parental empathy was associated with children’s attachment security, with perceptions of parental warmth, and with children’s emotional openness (Stern et al., 2015), highlighting the potential role of parental empathy in the development of healthy early parent–child relationships. Other researchers have found that different aspects of parental empathy (i.e., responsiveness to distress and warmth) predicted 6-to-8-year-old children’s social-emotional functioning: specifically, whereas mothers’ and fathers’ responsiveness to distress was a better predictor of regulation of negative emotions in children, mothers’ warmth was a better predictor of children’s regulation of positive emotions (Davidov & Grusec, 2006). Further, mothers’ responsiveness to distress predicted children’s prosocial reasoning and empathy in this study, suggesting that different features of parental empathy contribute to specific aspects of children’s social-emotional development.
In contrast, parents at high risk for physical child abuse have been found to exhibit a deficit in dispositional empathy, specifically obtaining lower scores on affective dimensions of empathy (e.g., empathic concern) (Perez-Albeniz & de Paul, 2003). Similarly, parents with higher self-reported affective and cognitive empathy have been found to have adolescent children with more (parent-reported) adaptive emotion regulation strategies, less emotion regulation difficulties (assessed by adolescents’ reports in a two-week daily diary), and lower levels of inflammation (i.e., C-reactive protein) (Manczak et al., 2015). Greater dispositional empathy in mothers has been found to be associated with better attentional focus and less proneness to anger in their 7-month-old infants (Kochanska et al., 2004), and with greater physiological arousal in their infants at 12–15 months of age in response to another infant’s emotions (Upshaw et al., 2015).
Despite evidence that caregiver empathy is important for child development, there are gaps in our understanding of its role in children’s social and emotional functioning. In particular, researchers have not examined the differential associations of dispositional and parental empathy with children’s early social-emotional development. Thus, we do not know whether caregivers’ dispositional empathy towards others and their parental empathy specifically for their child are dissociable constructs that have distinct associations with child functioning. Determining the relative effects of parental and dispositional empathy on child functioning is important in informing interventions focused on improving children’s caregiving environments, including whether dispositional or parental empathy should be targeted in efforts to support the caregiver–child relationship. In addition to determining whether these empathy constructs have distinct effects on child functioning, it is also important to examine whether dispositional and parental empathy have distinct neural substrates.
In this context, neuroimaging may be useful for investigating whether dispositional and parental empathy are dissociable constructs. Researchers have posited that caregiving behaviors are rooted in a “parental brain” network composed of neural regions that regulate social information, affective, and pain processing (Feldman, 2015; Kim et al., 2016; Young et al., 2017). Given the important role of empathy in caregiving, it is not surprising that these regions of the parental brain network overlap with areas that have been implicated in empathic processes. For example, Endendijk et al. (2020) found that higher self-reported nurturance in mothers was associated with greater activation in the amygdala and putamen in response to infant faces. Indeed, early motherhood may be a key window during which to assess the neural correlates of empathy, given that the first months of motherhood are characterized by alterations in brain structure and function (Dufford et al., 2019; Kim et al., 2010), some of which have been associated with positive maternal caregiving (Kim et al., 2010; 2011). For instance, at 3 months postpartum, mothers’ amygdala response to images of their own infant displaying positive affect was correlated with more positive feelings about and attachment toward their infant (Barrett et al., 2012). Further, in the first year of motherhood, greater insula response to images of infants has been associated with mothers’ greater use of cognitive empathy processes (Lenzi et al., 2009). Although researchers have assessed empathy-related brain activation in relation to measures of dispositional empathy in mothers (Ho et al., 2014; Zhang et al., 2020), they have not generally considered measures of parental empathy (although see Abraham et al., 2016, 2018; Swain, 2011). Tasks administered in the scanner that tap specific aspects of empathy, such as observing others experiencing pain or engaging in mentalizing, could help to identify patterns of neural activation that distinguish mothers who are higher and lower in levels of both dispositional and parental empathy.
Dispositional and parental empathy are both comprised of various facets (e.g., cognitive and affective dimensions). Investigating the respective levels of cognitive and affective facets for both empathy forms (i.e., dispositional and parental) may uncover specific psychological processes driving potential associations between mothers’ empathy and children’s early social-emotional functioning, as well as what skills an intervention might target. Additionally, examining the neural correlates of cognitive and affective facets of dispositional and parental empathy may uncover distinct substrates underlying each form and thereby further disentangle the two forms of empathy. For instance, it may be the case that although dispositional and parental affective facets of empathy largely share neural correlates, distinct brain regions may underlie dispositional cognitive empathy, which involves taking others’ perspectives generally, and parental cognitive empathy, which involves taking the perspective of one’s own infant.
Evidence from neuroimaging work suggests that different aspects of empathy (e.g., cognitive and affective dimensions) have both shared and unique neural substrates. For example, social cognitive tasks activate the right temporoparietal junction (TPJ) (Cheng et al., 2010; Saxe & Powell, 2006), a region involved in dispositional cognitive empathy (e.g., mentalizing, perspective-taking) (Preckel et al., 2018; Schurz et al., 2014). In contrast, observing or imagining others in physical or emotional pain activates brain regions implicated in salience detection and arousal, such as the anterior cingulate cortex (ACC), insula, and amygdala (Lockwood, 2016; Marsh et al., 2013), which are related to affective facets of empathy. Greater dispositional affective empathy has been associated with greater ACC and insula response to a loved one receiving a painful stimulus (Singer et al., 2004). Both dispositional affective and cognitive empathy have also been associated with anterior insula response to observing others in painful situations (Li et al., 2020). Better mentalizing skills have been associated with greater right TPJ activation during theory of mind tasks; right TPJ activation, however, has also recently been found to be associated with greater affective empathy (Knight et al., 2019). In sum, whereas the insula and right TPJ have been implicated in cognitive and affective facets of empathy, the ACC and amygdala are more frequently associated with affective facets of empathy.
As such, although parents’ dispositional and parental empathy have both been associated with children’s social-emotional functioning, the relative role of each is not well understood. Few studies have compared the effects of dispositional and parental empathy on developmental outcomes. We expected that there will be overlap in the brain regions associated with both parental and dispositional empathy, but we also expected that different forms of empathy will be related to activation in distinct brain regions during different empathy-related processes. Although the movie-watching task presents unfamiliar characters, the task allows for a consideration of neural activation in the context of different empathy-related processes, including pain processing and mentalizing. For example, given that parent–child relationships and interactions are often characterized by a high degree of self-other overlap (Reindl et al., 2018; Tan et al., 2015), activation in regions such as the anterior insula while observing others endure pain may be more closely related to parental than to dispositional empathy. In contrast, dispositional cognitive empathy may engage canonical mentalizing brain regions activated when individuals consider the perspective of others (e.g., right TPJ, ACC). Finally, taking the perspective of one’s infant, who is more cognitively immature than the typical targets of dispositional empathy, may recruit mentalizing regions to a lesser degree.
We had four aims in this study. First, we investigated the association between mothers’ dispositional and parental empathy (in relation to the focal child) when their infants were six months of age. Based on previous research (e.g., Salo et al., 2020), we hypothesized that these two forms of empathy will be moderately intercorrelated. Second, we examined whether mothers’ dispositional and parental empathy, assessed when their infant was 6 months old, were associated with their child’s social-emotional functioning as a toddler (~12 months later). Although we expected that both forms of empathy will be associated with toddler functioning, we hypothesized that parental empathy will be more strongly positively associated with toddlers’ social-emotional competencies and more strongly negatively associated with toddlers’ social-emotional problems than will dispositional empathy. Third, we tested the relative contributions of specific aspects of mothers’ dispositional and parental empathy (i.e., affective and cognitive facets) to toddlers’ social-emotional functioning. Finally, with a subset of the mothers, we explored the neural correlates of dispositional and parental empathy, including the affective and cognitive facets of each form of empathy. We used a passive movie-watching task to assess mothers’ neural activation in response to characters experiencing different belief states (“mentalizing” scenes)—related to cognitive empathy—and depictions of physical pain (“pain” scenes)—related to affective empathy. We focused our analysis on brain regions that have been linked to empathy in prior studies (Feldman, 2015; Kim et al., 2016; Young et al., 2017): right TPJ, bilateral ACC, insula, and amygdala.
Methods
Participants and Procedure
We recruited 155 mother and their infants (mean infant age=6.14 ± 0.43 months) to participate in the Brain and Behavior Infant Experiences Study (BABIES), an observational longitudinal study of the association between perinatal experiences and infant and toddler psychobiological development (Humphreys et al., 2018; King et al., 2021). For the current study, dyads with complete mother-reported data were included in the analyses of mothers’ empathy and toddlers’ social-emotional outcomes (N=118). To address the issue of missing data and leverage all cases with empathy data at T1, we conducted additional regression analyses using full information maximum likelihood (FIML) estimation. This set of analyses was run using the lavaan package in R (Rosseel, 2012). Further, we scanned a subset of these mothers (N=20 with usable data) to conduct our functional magnetic resonance imaging (fMRI) analyses of mothers’ empathy. Detailed participant characteristics are presented in Table 1.
Table 1.
Study Sample Characteristics
| Empathy analysis (N=118) | Included in fMRI analysis (N=20) | ||
|---|---|---|---|
|
| |||
| Demographics | |||
| Mother age, mean ± SD years | 33.51 ± 4.47 | 32.12 ± 3.91 | |
| Mothers’ race | |||
| White | 75 | 13 | |
| Asian American | 27 | 5 | |
| Black/African American | 3 | 0 | |
| Native Hawaiian/Pacific Islander | 3 | 0 | |
| American Indian or Alaska Native | 0 | 0 | |
| Other/More than One Race | 10 | 2 | |
| Mothers’ ethnicity | |||
| Hispanic or Latina/x | 17 | 4 | |
| Not Hispanic or Latina/x | 101 | 16 | |
| Mothers’ education | |||
| Some high school | 0 | 0 | |
| High school diploma/GED | 1 | 0 | |
| Some college, no degree | 7 | 1 | |
| Associate degree | 3 | 1 | |
| Trade/technical school | 3 | 0 | |
| Bachelor’s degree | 38 | 9 | |
| Graduate degree | 66 | 9 | |
| Annual household income | |||
| Less than $5,000 | 0 | 0 | |
| $5,001–15,000 | 1 | 0 | |
| $15,001–30,000 | 4 | 1 | |
| $30,001–60,000 | 14 | 2 | |
| $60,001–90,000 | 9 | 1 | |
| $90,001–150,000 | 32 | 4 | |
| More than $150,000 | 57 | 12 | |
| Decline to state/missing | 2 | 0 | |
| Infant age, mean ± SD months | 6.13 ± 0.43 | 6.04 ± 0.32 | |
| Infant race | |||
| White | 71 | 12 | |
| Asian American | 24 | 5 | |
| Black/African American | 3 | 1 | |
| Native Hawaiian/Pacific Islander | 1 | 0 | |
| American Indian or Alaska Native | 0 | 0 | |
| Other/More than One Race | 91 | 2 | |
| Infant ethnicity | |||
| Hispanic or Latina/o/x | 21 | 4 | |
| Not Hispanic or Latina/o/x | 96 | 16 | |
| Decline to state | 1 | 0 | |
| Infant sex | |||
| Male | 57 | 11 | |
| Female | 61 | 9 | |
| Scales | |||
| IBQ-R-SF | |||
| Negative emotionality | 3.10 ± 0.74 | 3.03 ± 0.70 | |
| IRI | |||
| Perspective-taking | 19.78 ± 3.91 | 19.40 ± 4.68 | |
| Empathic concern | 21.55 ± 3.81 | 20.65 ± 3.95 | |
| Total dispositional empathy | 41.33 ± 6.20 | 40.05 ± 6.90 | |
| PEM | |||
| Cognitive empathy | 63.31 ± 5.39 | 63.55 ± 5.42 | |
| Affective empathy | 47.79 ± 4.47 | 48.35 ± 4.46 | |
| Total parental empathy | 111.17 ± 8.10 | 111.85 ± 7.96 | |
| ITSEA | |||
| Competencies | 4.10 ± 0.79 | 4.23 ± 0.74 | |
| Problems | 1.73 ± 0.91 | 1.43 ± 0.52 | |
Time 1.
At Time 1, when infants were approximately 6 months of age, 142 of the 155 mothers who were recruited reported their dispositional and parental empathy (see below). Mothers who expressed interest in participating in the MRI scan session were screened for eligibility and excluded for MRI contraindications, left-handedness, certain medical diagnoses or events that may affect cerebral blood flow, neurovascular coupling, or the hemodynamic response (i.e., cancer, stroke, head injury with loss of consciousness or concussion, untreated migraine headaches, diabetes requiring insulin treatment, chronic kidney or liver disease, or neurological disorders) (Pak et al., 2017; Veldsman et al., 2014; Woods et al., 1994; Chen et al., 2014; Li et al., 2019; Zafiris et al., 2004; Tang et al., 2017; Levin et al., 1992), use of psychotropic (Schleim & Roiser, 2009), glucocorticoid (Lovallo et al., 2010), or hypolipidemic medications (Roca et al., 1981), and conditions affecting cerebral blood flow and metabolism (e.g., hypertension) (Xia et al., 2015). Eligible mothers were asked to refrain from caffeine consumption (Yang et al., 2018) and pain relievers (Zonta et al., 2002) 24 hours prior to the scan. MRI data were collected at the Lucas Center for Imaging at Stanford University.
Forty mothers participated in the MRI scan session. Mothers who were scanned did not differ from mothers who were not scanned in parental or dispositional empathy, toddler competencies or problems, infant negative emotionality, mothers’ age, race, ethnicity, education, or income (ps≥.053); however, mothers who were scanned were more likely to have a male infant enrolled in the study (p=.010). Of the 40 mothers who were scanned, 11 were excluded from the neuroimaging analyses due to scanner technical difficulties, 6 for poor quality data (inability to align the anatomical scan with the functional images during preprocessing), 1 for having previously seen the short movie, 1 for falling asleep during the task, and 1 for being left-handed. Scanned mothers with usable neuroimaging data did not significantly differ from scanned mothers without usable neuroimaging data in mother/infant race, ethnicity, age, mothers’ education, annual income, dispositional empathy, parental empathy, or infants’ temperament (i.e., negative emotionality) (ps>.176).
Time 2.
Approximately 1 year after Time 1, 118 of the 142 mothers from whom we obtained empathy data at Time 1 completed an online follow-up assessment in which they reported on their toddlers’ social-emotional functioning. Mothers who participated at Time 2 did not differ significantly from mothers who did not participate in toddler age or sex, or mothers’ age, ethnicity, education, income, or dispositional or parental empathy (ps>.093); however, mothers who participated at Time 2 were more likely to identify as White (p=.033) and to have infants who were lower in negative emotionality at Time 1 (p=.026).
Measures
Dispositional Empathy
At Time 1, mothers completed the Interpersonal Reactivity Index (IRI) to assess dispositional empathy (Davis, 1983). The IRI has good test-retest reliability and converges well with other empathy measures (Davis, 1980). We used the perspective-taking (e.g., “I sometimes find it difficult to see things from the other guy’s point of view” [reverse-scored]) and empathic concern (e.g., “I often have tender, concerned feelings for people less fortunate than me”) subscales to assess dispositional cognitive and affective empathy, respectively. Participants rated each of the statements on a scale from 0 (Does not describe me very well) to 4 (Describes me very well). Each subscale includes seven items that were summed to yield scores for dispositional cognitive and affective empathy. We summed the perspective-taking and empathic concern subscale scores to generate a total dispositional empathy score. In our sample internal reliability was acceptable for both dispositional empathy subscales and for the total score (Cronbach’s αs=.73-.78).
Parental Empathy
Mothers reported their parental empathy using the Parental Empathy Measure (PEM; Stern et al., 2015). The PEM consists of 25-items concerning participants’ feelings and thoughts about their child. Mothers rated items on a scale ranging from 1 (Not true at all) to 5 (Very true). The measure includes a 14-item subscale assessing parental cognitive empathy (e.g., “When my child is happy, I can understand why”) and an 11-item subscale assessing parental affective empathy (e.g., “When my child is upset, I feel concern for him/her”) as well as a total parental empathy score (the sum of the two subscale scores). In our sample, internal reliability was acceptable for the parental empathy subscales and for the total score (Cronbach’s αs =.73-.81).
Infant Temperament
Mothers reported on their infant’s temperament using the Infant Behavior Questionnaire-Revised Short Form (IBQ-R-SF), a caregiver report measure of infant temperament validated for infants 3–12 months of age (Gartstein & Rothbart, 2013; Putnam et al., 2014). The IBQ-R-SF consists of 91 items on which parents rate the extent to which their infant exhibited various behaviors over the past 7 days on a Likert scale ranging from 1 (Never) to 7 (Always) and a separate choice for Does not apply. The IBQ-R-SF includes subscales of surgency/extraversion, negative emotionality, and orienting/regulatory capacity. In the present study we focused on the domain of negative emotionality (e.g., “When tired, how often did the baby show distress?”) to include as a covariate in regression analyses given the documented associations of this domain with psychopathology in later life (Kostyrka-Allchorne et al., 2020). In our sample internal reliability was acceptable for the IBQ-R-SF negative emotionality subscale (Cronbach’s α=.83).
Toddler Social-Emotional Competencies and Problems
Mothers completed an abbreviated version of the Infant–Toddler Social and Emotional Assessment (ITSEA; Carter et al., 2003). The ITSEA includes several subscales across four domains (internalizing, externalizing, dysregulation, and competence) and asks parents to rate the extent to which their child exhibits behaviors on a scale from 0 (Not true/rarely) to 2 (Very true/often). In the present study, we administered eight of the ITSEA subscales and computed a total score for social-emotional problems by summing the mean scores for internalizing (depression/withdrawal, general anxiety), externalizing (activity/impulsivity, aggression, defiance), and negative emotionality. We computed a total score for social-emotional competencies by summing the mean scores for the play, social-relatedness, and empathy subscales. Twenty-one percent of toddlers in our sample met the age- and gender-normed cutoffs for clinical concern. In our sample internal reliability was acceptable for the social-emotional problems and competencies variables (Cronbach’s αs=.88 and .78, respectively).
Passive Animated Movie fMRI Task
Participants watched the animated short movie “Partly Cloudy” (Pixar Animation Studios) while undergoing functional MRI (fMRI) scanning (total movie time=5 min 36 sec). This short movie has been used to localize brain regions implicated in social cognitive processes (Jacoby et al., 2016), and movie events have been coded into 4 categories: “Control,” “Mental,” “Social,” and “Pain”). Briefly, “Control” events were characterized by scenes without specific character-related events (e.g., scenes with birds flying; 3 events, 24 seconds total); “Mental” events induced the viewer to think about a character’s thoughts (e.g., a character falsely believing he has been abandoned by his companions; 4 events, 44 seconds total); “Social” events involved characters interacting without engaging mental/emotional representation (e.g., stork and cloud playing; 5 events, 28 seconds); and “Pain” events depicted a character experiencing physical pain (e.g., character bitten by crocodile; 7 events, 26 seconds total). The remainder of the movie was not coded for scenes and, therefore, was not included in analyses, resulting in a total of 122 seconds of coded scenes.
fMRI Acquisition, Preprocessing, and Cluster Correction
MRI scans were conducted on a GE Discovery MR750 scanner (GE Medical Systems, Milwaukee, WI) equipped with a 32-channel head coil (Nova Medical). We collected fast spoiled gradient echo (FSPGR) T1-weighted sagittal anatomical images (repetition time [TR]=8.2 ms, slice thickness=1, scan time=5 min 6 sec) to be used for alignment and registration of functional images. Task-based blood oxygen level dependent (BOLD) fMRI data were acquired using T2*-weighted oblique slices aligned to the anterior and posterior commissure (TR=2000 ms, echo time [TE]=30 ms, flip angle=77°, voxel size=.90 mm3, slices=32, slice thickness=4.0 mm, scan time=4 min 50 sec). We applied higher-order shims prior to the movie-watching task to decrease magnetic field inhomogeneities (Kim et al., 2002).
All functional images were preprocessed and analyzed in AFNI (Cox, 1996). Preprocessing included slice timing and motion correction, alignment to anatomical images, removal of first 5 TRs to allow for magnet stabilization (blank screen before start of movie), spatial normalization (MNI 152T1), spatial blurring (5mm FWHM isotopic Gaussian kernel), and censoring volumes with head motion greater than 0.5 mm from the previous volume. Any participant with more than 20% of their volumes censored would be dropped from analyses; however, no participant met this cutoff threshold.
Regions of Interest
We examined associations of the empathy measures with BOLD fMRI signal during mentalizing (Mental>Control) and pain (Pain>Control) contrasts in regions of interest (ROIs) based on previous findings (i.e., ACC, right TPJ, insula, and amygdala) (Decety, 2015; Lockwood, 2016). We created the amygdala ROI mask using the Brainnetome atlas (Fan et al., 2016), the ACC and insula masks using the Eickhoff-Zilles macro labels N27 atlas (Eickhoff et al., 2005, 2006, 2007), and the right TPJ mask using the MNI Glasser HCP atlas (Glasser et al., 2016) by combining the three temporo-pariento-occipital junction ROIs. We resampled ROIs to subject space prior to analyses and used AFNI’s 3dmaskave to extract beta parameters from each ROI for both contrasts of interest. ROI masks are presented in Figure 1.
Figure 1.
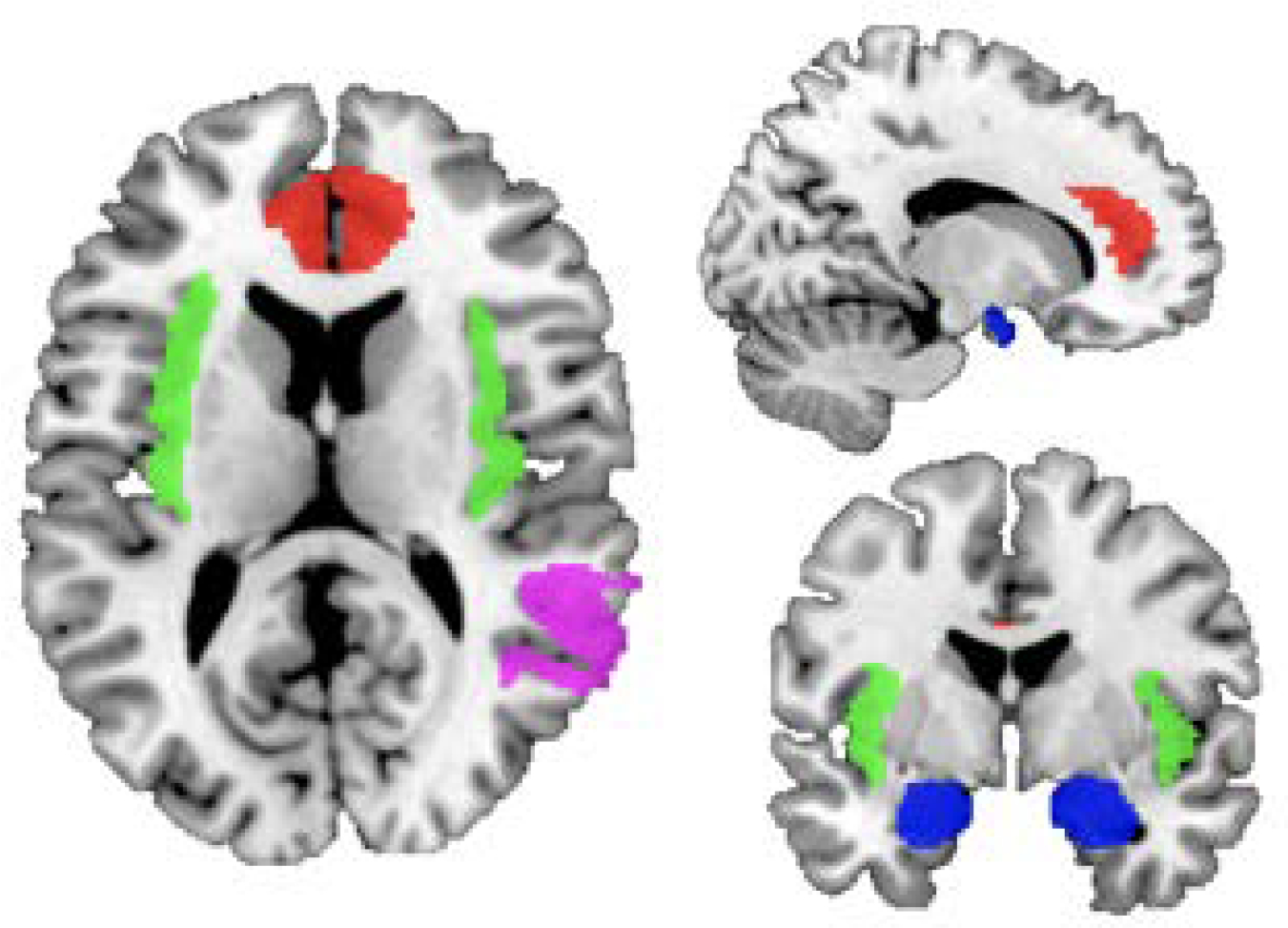
Regions of interest. Labels: red, anterior cingulate cortex; green, insula; violet, right temporoparietal junction; blue, amygdala.
Data Analysis
First, we conducted Pearson’s correlations to examine the associations among mothers’ total dispositional and parental empathy scores and toddlers’ social-emotional competencies and problems. Second, we conducted ordinary least squares (OLS) regression analyses to test whether mothers’ empathy was associated with toddlers’ social-emotional outcomes (i.e., competencies and problems) after adjusting for covariates. In the first set of linear regression models, we included dispositional and parental empathy to test whether one form of empathy was significantly associated with toddlers’ outcomes after controlling for the other form. We included infant temperament (negative emotionality) as a covariate. In the second set of models, we included all four empathy subscales in the model (i.e., parental cognitive empathy, parental affective empathy, dispositional cognitive empathy, dispositional affective empathy) along with infant temperament to examine whether specific aspects of empathy were associated with toddler functioning. In addition, we conducted sensitivity analyses for Models 1–4 in which we included a measure of socioeconomical status (SES) (specifically, income-to-needs ratio) and child sex as additional covariates. We corrected for multiple comparisons by setting the threshold for statistical significance for these analyses at p<.025 (.05/2) to account for the two a priori tests. Finally, we conducted Pearson’s correlations to explore potential associations between ROI activations (i.e., bilateral ACC, amygdala, insula, and right TPJ) during the two movie contrasts probing cognitive and affective empathy (i.e., Mental>Control, Pain>Control), respectively, and mothers’ dispositional and parental empathy and their facets. All statistical tests were performed in R v. 4.0.3 (R Core Team, 2020).
Results
Correlations Among the Measures of Maternal Empathy and Offspring Functioning
Correlations among measures of mothers’ empathy, infant temperament, and toddler social-emotional competencies and problems are presented in Table 2. As we hypothesized, mothers’ dispositional empathy was significantly correlated with their parental empathy (r=.44, p<.001, 95%CI[0.28, 0.58]). Higher self-reported dispositional and parental empathy in mothers at Time 1 was associated with more social-emotional competencies (r=.21, p=.022, 95%CI[0.03, 0.38], and r=.29, p=.001, 95%CI[0.12, 0.45], respectively) and fewer social-emotional problems in toddlers at Time 2 (r=−.21, p=.023, 95%CI[−0.38, −0.03], and r=−.34, p<.001, 95%CI[−0.49, −0.17], respectively).
Table 2.
Zero-Order Correlations Between Mothers’ Empathy, Infant Temperament, and Toddler Outcomes Variables.
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 1. Dispositional empathy (total) | .81 *** | .80 *** | .44 *** | .35 *** | .42 *** | .21 * | −.21 * | −.07 |
| 2. Dispositional cognitive empathy | .29 ** | .25 ** | .18 | .27 ** | .15 | −.22 * | .04 | |
| 3. Dispositional affective empathy | .46 *** | .38 *** | .40 *** | .19 * | −.11 | −.15 | ||
| 4. Parental empathy (total) | .84 *** | .85 *** | .29 ** | −.34 *** | −.25 ** | |||
| 5. Parental cognitive empathy | .44 *** | .24 ** | −.41 *** | −.31 *** | ||||
| 6. Parental affective empathy | .26 *** | −.20 * | −.12 | |||||
| 7. ITSEA competencies | −.14 | −.11 | ||||||
| 8. ITSEA problems | .26 ** | |||||||
| 9. Infant negative emotionality | ||||||||
Note.
p<.001
p<.01
p<.05.
Values reported are Pearson’s correlation coefficients.
Given that the empathy measures are intercorrelated, we tested for potential multicollinearity issues using variance inflation factor (VIF) values. All VIF values were < 1.4, suggesting small to moderate correlations among predictor variables; these were not large enough (i.e., VIF>5) to raise concerns of multicollinearity. The VIFs from Model 3 are: total parental empathy: 1.324; total dispositional empathy: 1.247. The VIFs from Model 4 are: parental cognitive empathy: 1.435; parental affective empathy: 1.382; dispositional cognitive empathy: 1.141; dispositional affective empathy: 1.382.
Mothers’ Total Dispositional and Parental Empathy and Toddlers’ Social-Emotional Functioning
After controlling for mothers’ dispositional empathy and infant temperament at Time 1, OLS linear regressions revealed that higher parental empathy in mothers was significantly associated both with more social-emotional competencies (β=.233, p=.025) and with fewer problems (β=−.028, p=.012) in toddlers at Time 2. In contrast, when controlling for mothers’ parental empathy and infant temperament, dispositional empathy was not significantly associated with either toddlers’ competencies or problems (ps>.05). See Table 3 for detailed statistics of these analyses.
Table 3.
Regression Models Testing Mothers’ Dispositional and Parental Empathy at Time 1 Predicting Toddlers’ Social-Emotional Competencies and Problems at Time 2
| Model 1 Predicting Social-Emotional Competencies | B | SE | β | 95%CI | p |
|
| |||||
| Intercept | 1.141 | 1.120 | <.001 | [−.18, .18] | >.999 |
| Infant Negative Emotionality | −.043 | .099 | −.040 | [−.22, .14] | .663 |
| Dispositional Empathy | .014 | .013 | .106 | [−.09, .30] | .290 |
| Parental Empathy | .023 | .010 | .233 | [.03, .44] | .025 |
|
| |||||
| Model 2 Predicting Social-Emotional Problems | B | SE | β | 95%CI | p |
|
| |||||
| Intercept | 4.670 | 1.237 | <.001 | [−.17, .17] | >.999 |
| Infant Negative Emotionality | .233 | .109 | .191 | [.01, .37] | .035 |
| Dispositional Empathy | −.012 | .014 | −.085 | [−.28, .11] | .378 |
| Parental Empathy | −.028 | .011 | −.253 | [−.45, −.06] | .012 |
Note. B, unstandardized beta. SE, standard error associated with unstandardized beta. β, standardized beta. p, p-value. Bolded p-values indicate significant associations after correcting for multiple comparisons.
Mothers’ Cognitive and Affective Empathy and Toddlers’ Social-Emotional Outcomes
We conducted exploratory linear regression analyses to examine whether cognitive or affective facets of parental and dispositional empathy were associated with toddler outcomes by including all four empathy subscales, assessed at Time 1 (and infant temperament as a covariate), in two models: one examining toddlers’ social-emotional competencies and the other examining toddlers’ problems. No dispositional empathy subscale was associated with either toddlers’ competencies or problems following multiple comparisons correction (ps≥.033). Higher self-reported parental cognitive empathy in mothers predicted fewer social-emotional problems in toddlers (β=−.060, p<.001); no other parental empathy subscale was associated with either competencies or problems (ps>.05). Scatterplots of the unadjusted correlations between mothers’ parental empathy and toddlers’ social-emotional competencies and problems are presented in Figure 2. Detailed statistics from these models are presented in Table 4.
Figure 2.
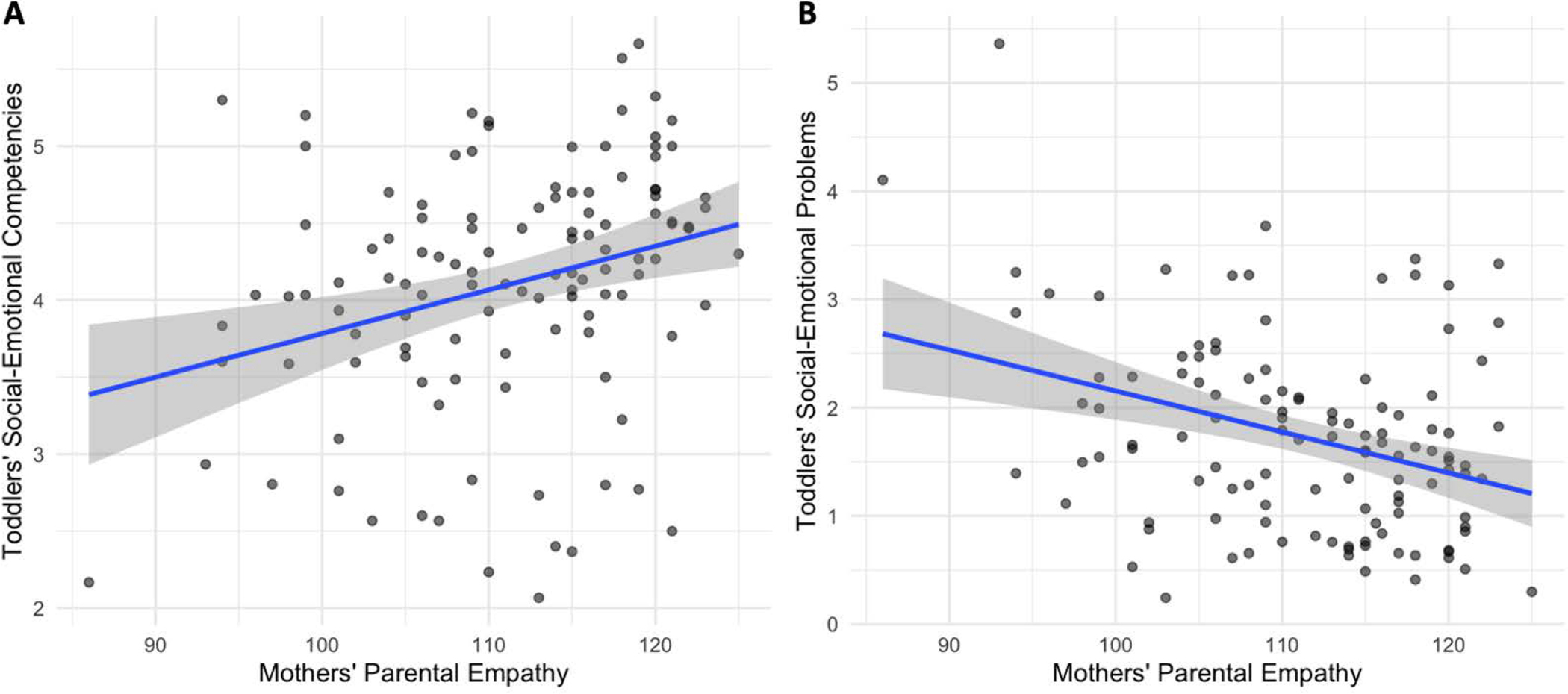
Scatterplots of mothers’ total parental empathy at Time 1 and toddlers’ social-emotional (a) competencies and (b) problems at Time 2.
Table 4.
Regression Models Testing Mothers’ Cognitive and Affective Empathy at Time 1 Predicting Toddlers’ Social-Emotional Competencies and Problems at Time 2
| Model 3 Predicting Social-Emotional Competencies | B | SE | β | 95%CI | p |
|
| |||||
| Intercept | 1.186 | 1.111 | <.001 | [−.18, .18] | >.999 |
| Infant Negative Emotionality | −.045 | .102 | −.042 | [−.23,.15] | .658 |
| Dispositional Cognitive Empathy | .015 | .019 | .075 | [−.11, .15] | .434 |
| Dispositional Affective Empathy | .010 | .022 | .047 | [−.16, .25] | .651 |
| Parental Cognitive Empathy | .019 | .016 | .131 | [−.08, .34] | .227 |
| Parental Affective Empathy | .028 | .019 | .156 | [−.05, .37] | .142 |
|
| |||||
| Model 4 Predicting Social-Emotional Problems | B | SE | β | 95%CI | p |
|
| |||||
| Intercept | 5.349 | 1.178 | <.001 | [−.16, .16] | >.999 |
| Infant Negative Emotionality | .209 | .108 | .171 | [.00, .35] | .056 |
| Dispositional Cognitive Empathy | −.045 | .021 | −.192 | [−.37, −.02] | .033 |
| Dispositional Affective Empathy | .026 | .023 | .110 | [−.08, .30] | .256 |
| Parental Cognitive Empathy | −.060 | .017 | −.355 | [−.55, −.16] | <.001 |
| Parental Affective Empathy | −.004 | .020 | −.017 | [−.21, .18] | .859 |
Note. B, unstandardized beta. SE, standard error associated with unstandardized beta. β, standardized beta. p, p-value. Bolded p-values indicate significant associations after correcting for multiple comparisons.
Full information maximum likelihood (FIML) approach
Using the FIML approach for missing data, we found that total parental empathy remained prospectively associated with toddlers’ social-emotional competencies (β=.02, p=.013) and problems (β=−.02, p=.017) after covarying infant temperament and mothers’ total dispositional empathy; however, mothers’ total dispositional empathy was not associated with either toddlers’ social-emotional competencies (β=.01, p=.345) or problems (β=−0.01, p=.399) after covarying total parental empathy. Neither the cognitive nor the affective empathy subscale for either dispositional or parental empathy was associated with toddlers’ competencies after covarying for all other empathy subscales and infant temperament (all ps>.123). This was similarly the case when considering toddlers’ problems as the outcome variable of interest (all ps>.099), with the exception of mothers’ parental cognitive empathy (β=−.05, p<.001): greater parental cognitive empathy was prospectively associated with fewer social-emotional problems in toddlers after controlling for the other empathy subscales and infant temperament, consistent with our primary findings in the sample with complete data.
Sensitivity Analyses
Detailed results from the post hoc sensitivity analyses are presented in the Supplement. Briefly, after including SES and child sex as additional covariates, neither mothers’ total dispositional nor parental empathy was associated with either toddlers’ social-emotional competencies or problems (all ps>.075). No empathy subscale was significantly associated with toddlers’ competencies (all ps>.105). Similarly, neither dispositional nor parental affective empathy was associated with toddlers’ competencies; however, both mothers’ dispositional and parental cognitive empathy were prospectively associated with fewer social-emotional problems in toddlers (ps=.021 and .015, respectively). All models included other empathy measures and infant temperament as covariates as in the primary analyses. See Tables S1 and S2 in the Supplement for detailed model statistics.
Neural Correlates of Mothers’ Empathy During Mentalizing and Pain Scenes
Higher total parental empathy in mothers was associated with greater right TPJ activation during pain scenes (r=.59, p=.006, 95%CI[0.19, 0.99]); no other association between mothers’ total dispositional or parental empathy and ROI activation during mentalizing or pain scenes was significant (rs≤.38, ps>.05).
When we examined specific facets of each form of empathy, we found that higher dispositional cognitive empathy was associated with greater activation during mentalizing scenes in the bilateral ACC (r=.50, p=.025, 95%CI[0.07, 0.93]) and insula (r=.45, p=.048, 95%CI[0.00, 0.89]). In addition, higher parental affective empathy was associated with greater bilateral insula activation during mentalizing scenes (r=.51, p=.021, 95%CI[0.09, 0.94]) and with greater bilateral insula (r=.48, p=.034, 95%CI[0.04, 0.91]) and right TPJ (r=.58, p=.008, 95%CI[0.17, 0.98]) activation during pain scenes. No other empathy subscale was associated with ROI activation during either contrast (rs≤.42, ps>.05).
Discussion
The present study was designed to investigate whether mothers’ parental (child-specific) empathy is dissociable from mothers’ dispositional (other-specific) empathy by examining whether the two constructs have distinct prospective associations with children’s social and emotional functioning and whether they are associated with distinct neural correlates. In a sample of 118 mother–infant dyads, we found that dispositional and parental forms of empathy in mothers are moderately intercorrelated. Mothers’ parental empathy when their infants are 6-months-old predicts toddlers’ social-emotional functioning one year later, controlling for dispositional empathy and infant temperament. Higher levels of mothers’ parental cognitive empathy, specifically, predicts fewer social-emotional problems. Finally, dispositional and parental empathy have both unique and shared patterns of brain activation in the bilateral insula, ACC, and right TPJ during a passive movie-watching task involving mentalizing and pain scenes.
Prior studies have found that individual differences in mothers’ empathy and empathy-related constructs are associated with children’s social-emotional outcomes (e.g., Kochanska et al. 2004; Manczak et al., 2016); however, much of this research has focused on mothers’ dispositional empathy and has not distinguished this general form of empathy from mothers’ (parental) empathy towards their own child. Our findings indicate that empathy specific to one’s child is a stronger predictor of toddlers’ social-emotional outcomes than is dispositional empathy. Although both higher dispositional and parental cognitive empathy were initially associated with fewer social-emotional problems, only the latter survived multiple comparisons correction and had a larger effect (dispositional cognitive empathy: β=−.045; parental cognitive empathy: β=−.060). Our results highlight the role that parental cognitive empathy, in particular, plays in protecting toddlers from developing social-emotional difficulties, and, as such, may represent a specific target for early interventions.
It is important to ask why mothers’ parental empathy is associated more strongly with toddlers’ social-emotional outcomes than is their dispositional empathy. Consistent with past work (Salo et al., 2020), we found that dispositional and parental empathy are only moderately correlated. One clear distinction is the target of empathy—by definition, parental empathy focuses on one’s own infant, which requires sensitivity and attunement to infant cues. In contrast, dispositional empathy calls for one to empathize with others more generally, which may require different psychological processes. Caregivers must accurately identify and address an infant’s interests, desires, or needs; those who are more sensitive to these needs (e.g., who are able to take the perspective of the infant) may be more likely to meet the infant’s needs and foster a relationship that benefits the child’s social-emotional development (Leerkes, 2010; van den Boom, 1994).
With respect to parental empathy, we found that after controlling for infant temperament, mothers’ parental cognitive empathy was more strongly associated with toddler outcomes (i.e., social-emotional problems) than was mothers’ parental affective empathy. In previous work, researchers have found that mothers’ “parental mind-mindedness,” or tendency to view their child as a mental agent with thoughts, feelings, and desires during the first several months of life (Meins et al., 2003), is associated with better executive functioning at 18 and 26 months (Bernier et al., 2010) and with fewer behavioral difficulties at 44 and 61 months (Meins et al., 2013). Cognitive empathy processes are posited to be one of the key mechanisms supporting caregiving behaviors across different relationships (Batson, 1991). Parenting styles and behaviors that are implicated in early social-emotional development, such as mothers’ sensitivity to infant distress (Leerkes et al., 2009), may distinguish mothers who regularly engage in theorizing about the minds of their infants at this early age from mothers who do not; however, this hypothesis would require explicit testing. Finally, parents who reflect on the thoughts, feelings, and motives that underlie their infant’s behaviors may also be more likely to have securely attached children (Meins et al., 2001; Stern et al., 2015; Symons & Clark, 2000), which may mediate the relation between parental cognitive empathy and subsequent social-emotional adjustment. Taken together, our findings build on previous research to underscore the distinct and important role of parental empathy during an infant’s first year of life in promoting healthy social-emotional development in toddlerhood.
An exploratory aim of this study was to leverage an fMRI movie-watching task to investigate the neural correlates of dispositional and parental empathy in mothers. Researchers have found that individual differences in mothers’ empathy and empathy-related processes are associated with neural activation in regions implicated in salience processing and social cognition (Elmadih et al., 2016; Lenzi et al., 2009). Although most neuroimaging studies with mothers have focused on neural responses to infant cues, such as facial and vocalic expressions (Bornstein et al., 2017; Lenzi et al., 2009; Zhang et al., 2020), we implemented a movie-watching task that has previously been used as an empathy-localizer in order to assess neural responses to scenes that depicted mentalizing processes or physical pain to probe specific dimensions of empathy (i.e., cognitive and affective processes, respectively). Importantly, the movie includes animated, non-human, unfamiliar characters that likely differ in important ways from the typical targets of empathy in everyday life. Nevertheless, the movie task in question has been validated against the most commonly used empathy tasks and has been found to reliably recruit (i.e., “localize”) empathy-related brain networks (Jacoby et al., 2016). Therefore, although the fMRI task does not include infants or other human characters, there is evidence to support use of this task to investigate brain regions associated with empathy-related processes.
Interestingly, we found that higher total parental empathy in mothers was associated with greater right TPJ activation during pain scenes, which appeared to be driven by parental affective empathy, given that activation in this ROI was not associated with cognitive empathy. Although the right TPJ is typically regarded as a region central to cognitive empathy, recent studies have also implicated right TPJ functioning in affective empathy processes (Knight et al., 2019; Miller et al., 2020). It is possible that right TPJ activation is related differentially to facets of empathy across development; that is, right TPJ activation may underlie affective empathy processes directed toward infants but, over time, may also contribute to cognitive processes as the target of empathy develops cognitive processes with which the empathizer might relate. Notably, right TPJ activation was not associated with total dispositional empathy or its facets in our sample.
During both mentalizing and pain scenes, greater bilateral insula activation was associated with higher parental affective empathy, and with higher dispositional cognitive empathy during mentalizing scenes. Given the involvement of this region in salience processing (Menon & Uddin, 2010), our results suggest that the insula broadly supports different forms and facets of empathy. This is consistent with research showing that mothers who demonstrated greater ability to represent others’ mental states (i.e., cognitive empathy) also had greater insula activation in response to images of infants’ emotional faces (Lenzi et al., 2009), implicating this structure in cognitive and affective processes when considering both others in general and infants specifically. Previous studies have also reported greater insula activation when observing others in pain (Lockwood, 2016; Singer et al., 2004). It is important to note, however, that the insula is a large structure, and different insular subregions may support these various forms and facets of empathy.
Finally, although researchers have found that the ACC responds when participants observe others in pain (Li et al., 2020; Marsh et al., 2013; Singer et al., 2004), we found that greater ACC activation was associated with empathy (dispositional cognitive empathy, specifically) only during mentalizing scenes. A recent meta-analysis found that the ACC was not involved in mentalizing but was implicated in “grasping and sharing others’ emotional and sensory feelings,” which the study authors referred to as ‘empathy’ (Arioli, Cattaneo, Ricciardi, & Canessa, 2021). Although the ACC (in an empathy context) is typically associated with experiencing and witnessing pain (Morrison, Lloyd, Di Pellegrino, & Roberts, 2004), others have also described the ACC in the context of mentalizing (Frith & Frith, 2021; Zaki & Ochsner, 2012). Similar to the insula, the ACC is a large structure with various subregions that are associated with different cognitive, affective, and motivational processes (Botvinick, Cohen, & Carter, 2004; Bush, Luu, & Posner, 2000; Holroyd & Yeung, 2012). Thus, a study with a larger neuroimaging sample may be able to better differentiate the involvement of specific insula and ACC subregions in various forms and facets of empathy processes.
Taken together, our findings suggest that some brain regions involved in both forms of empathy are functionally dissociable. Whereas right TPJ activation was specific to parental empathy, the bilateral ACC was specific to dispositional empathy, lending support to the formulation that dispositional and parental empathy can be meaningfully differentiated. Conversely, bilateral insula activation was associated with both parental and dispositional empathy; thus, this region may play an important role in processes that are shared across different forms of empathy. It is important to note that these associations involving neuroimaging data are based on a much smaller sample than our behavioral analyses of mothers’ empathy and toddler social-emotional outcomes. Therefore, while informative, these neuroimaging findings and the corresponding interpretations should be viewed as preliminary.
We should note four limitations of this study. First, we used mother-report measures of parental empathy and toddler social-emotional outcomes, and shared method variance may have contributed to some of our findings; however, this would not explain stronger and weaker associations among these constructs in this study. It is important to note that our questionnaires assessed empathy at the trait-level (i.e., an ability or capacity that is largely stable across time), whereas the fMRI task measured neural activation that likely reflects or is related to state-level empathy or empathy-related processes (i.e., context-specific empathy engagement based on situational cues). Prior research suggests that empathy is composed of both state- and trait-level processes (see Clark et al., 2019, for review), and we assumed that there is some correspondence between processes at these two levels. For example, one study tracked state measures of empathy twice per week for up to 10 weeks and found that state and trait empathy were positively correlated, despite the fact that empathy assessed within-person varied both within and across days (Nezlek et al., 2001); thus, although empathic responses might be elicited by situational cues, state- and trait-level empathy appear to be related. The fMRI movie-watching task administered in the current study likely probed state-like empathy processes (given numerous scenes and contrasts with varying salient features), and it is plausible that these neural responses are related to and inform trait-level characterizations of empathy in participants, even if they are measured at the state level. Indeed, several prior studies have considered links between trait-level measures of empathy and neural activation in the context of empathy tasks (e.g., Bufalari & Ionta, 2013; Lockwood et al., 2015; Masten et al., 2011; Rameson et al., 2012). For example, Rameson and colleagues found that individuals with higher state-level empathy—based on self-report empathy diary entries that participants completed each day for two weeks—also had higher trait-level empathy and stronger mPFC recruitment when viewing sad images than did participants with lower state-level empathy (Rameson et al., 2012). Thus, although our fMRI movie-watching task likely assesses state-level empathy processes whereas the dispositional and parental empathy measures (IRI and PEM, respectively) are more closely aligned with trait-level characteristics, it is likely that there is some degree of overlap given research indicating correspondence between the two. There are some limitations of maternal reports of empathy and of children’s temperament and behavioral outcomes; thus, future studies may benefit from the inclusion of task-based, interview, and observational measures of mothers’ empathy (e.g., Leerkes et al., 2011; Stern et al., 2015) and other constructs to reduce risk of bias and issues related to shared method-variance. In addition, we recommend that researchers include measures of parenting behaviors to explore mechanisms by which parental cognitive empathy is linked to child outcomes. Finally, given the documented effects of adversity on emotion regulation and psychopathology, researchers might consider using measures like the Assessment of Parent and Child Adversity (APCA) to assess the effects of deleterious events at various stages of life in relation to both children’s and mothers’ mental health (King et al., 2022), as well as measures that assess specific social processes to characterize the caregiver–child relationship (King et al., 2021). Second, the participants in the sample were generally highly educated, high in socioeconomic status, and largely identified as White; it is not clear whether our findings generalize to more diverse samples, underscoring the need in future research to recruit diverse, representative study samples. Third, our study, like many others, focused on the mother as the primary caregiver of interest. Future research will benefit from considering other caregivers (e.g., fathers; childcare providers) in assessing the role of caregiver empathy in adult–infant relationships and subsequent child development. Finally, the relatively small sample size for our neuroimaging analysis limited our ability to detect more statistically robust links between mothers’ empathy and neural activation.
Despite these limitations, however, our study also had several notable strengths. First, we recruited a large sample and assessed multiple forms and facets of empathy (i.e., dispositional and child-directed; cognitive and affective dimensions). Second, the longitudinal design of our study enabled us to test prospective associations between mothers’ empathy when their infants were 6 months old with social-emotional competencies and problems in these children one year later, at 18 months of age. Third, we used multimodal approaches which largely converged with our hypothesis that dispositional and parental empathy in mothers are dissociable in terms of both behavioral outcomes in children and neural correlates of these forms of empathy. By using an empathy localizer fMRI task to examine relevant brain regions involved in social cognitive and affective processes, we were able to identify both shared and distinct neural correlates of dispositional and parental empathy in mothers. Finally, our findings raise the possibility of developing novel interventions. Specifically, although dispositional empathy has received considerable attention for its role in interpersonal relationships (e.g., Håkansson & Montgomery, 2003; Joireman, Needham, & Cummings, 2002; Reynolds & Scott, 1999), our findings underscore the importance of fostering infant-directed (parental) empathy, with an emphasis on cognitive dimensions, to promote healthy child development. One possible parental intervention, based on our findings of the prospective association between parental cognitive empathy and toddlers’ social-emotional functioning, might target reflective functioning—the capacity to understand/explain behaviors in the context of the actor’s mental states or intentions. One study of recent mothers recovering from substance use disorders found that greater capacity for self-mentalization (i.e., making sense of their own difficult emotions related to their parenting role) was associated with better social-emotional functioning in their toddlers (Suchman et al., 2010). Further, greater child-mentalization in mothers was associated with clearer cue signaling in children (e.g., widening of eyes, increased motor activity, recognizable arm movements, directed movements). Suchman et al. (2017) assessed mothers with a history of substance use disorders and documented the effects over one year of a 12-week randomized clinical trial of mentalization-based individual therapy versus a psychoeducation control comparison. Suchman et al. showed that mothers who received mentalization-based therapy exhibited several psychological benefits, including improvement in sensitivity, in quality of mother-child interactions, and in attachment status. Finally, Marvin and colleagues (2002) described an attachment intervention for parent-child dyads in which parents are taught to shift from a defensive, insecure caregiving strategy to one centered on empathy for one’s child. Taken together, these findings suggest that parental cognitive empathy exerts its supportive effects on children’s social-emotional development by mentalizing not only about the child’s internal state, but about one’s own states as well.
Our findings raise several possibilities for further studies, including testing hypotheses that investigate possible mechanisms linking mothers’ parental cognitive empathy to reduced risk of social-emotional problems, which may include infant-directed language use during early life. Recognizing and taking young infants’ perspectives likely plays an important role in supporting healthy social-emotional development during early life. In the present study we could not identify neural correlates that were specific to mothers’ parental cognitive empathy; this finding should be replicated and expanded upon in a larger study examining broader neural circuitry. Future investigations should also consider whether infants are differentially susceptible to the association between mothers’ empathy and social-emotional development. For example, infants with certain behavioral or psychological traits may be more sensitive to effects of relative presence or absence of maternal empathy, both for better and for worse. In addition, it is important to consider how an infant’s disposition may influence mothers’ caregiving behaviors, including engagement in empathy-related processes. For example, whereas some mothers may exhibit empathic caregiving with an infant high in soothability, other mothers might exhibit less sensitive caregiving and more personal distress in caring for an infant lower in soothability. Longitudinal studies that include repeated measures of parenting and infant temperament are necessary for considering such transactional processes.
Our behavioral and neuroimaging findings converge to suggest that although dispositional and parental empathy are related constructs that share neural correlates, they are also dissociable constructs that have different associations with children’s early social-emotional development. Taken together, our findings underscore the potential role of parental cognitive empathy during the first year of an infant’s life in protecting against social-emotional problems during toddlerhood, an important developmental period for the application of targeted interventions with young children at highest risk for social-emotional dysfunction.
Supplementary Material
Acknowledgements
We thank Anna Cichocki, Cheyenne Garcia, Fran Querdasi, Marissa Roth, Jill Segarra, and Lucinda Sisk for their assistance in data collection and management. We also thank the mothers and infants who participated in this study.
Funding Statement:
This research was supported by grants from the National Institutes of Health (R21HD090493 and R21MH111978 to IHG; T32GM081760 to AO; F32 HD105385 to LSK), a Stanford Child Health Research Institute New Idea Award, and by funding from the National Science Foundation (2042285) to KLH.
Footnotes
Conflicts of Interest: None
Ethics Statement: Research involving human participants was reviewed and approved by the Institution Review Board at Stanford University. Mothers provided written informed consent for themselves and their infants to participate in this study.
Data Availability:
The datasets used and analyzed in the current study will be made available by the corresponding author upon reasonable request.
References
- Abraham E, Hendler T, Zagoory-Sharon O, & Feldman R (2016). Network integrity of the parental brain in infancy supports the development of children’s social competencies. Social Cognitive and Affective Neuroscience, 11(11), 1707–1718. doi: 10.1093/scan/nsw090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham E, Raz G, Zagoory-Sharon O, & Feldman R (2018). Empathy networks in the parental brain and their long-term effects on children’s stress reactivity and behavior adaptation. Neuropsychologia, 116, 75–85. doi: 10.1016/j.neuropsychologia.2017.04.015 [DOI] [PubMed] [Google Scholar]
- Arioli M, Cattaneo Z, Ricciardi E, & Canessa N (2021). Overlapping and specific neural correlates for empathizing, affective mentalizing, and cognitive mentalizing: A coordinate-based meta-analytic study. Human Brain Mapping, 42(14), 4777–4804. doi: 10.1002/hbm.25570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J, Wonch KE, Gonzalez A, Ali N, Steiner M, Hall GB, & Fleming AS (2012). Maternal affect and quality of parenting experiences are related to amygdala response to infant faces. Social Neuroscience, 7(3), 252–268. doi: 10.1080/17470919.2011.609907 [DOI] [PubMed] [Google Scholar]
- Batson CD (1991). The altruism question: Toward a social-psychological answer. Hillsdale, NJ: Lawrence Erlbaum. doi: 10.4324/9781315808048 [DOI] [Google Scholar]
- Bernier A, Carlson SM, & Whipple N (2010). From external regulation to self-regulation: Early parenting precursors of young children’s executive functioning. Child Development, 81(1), 326–339. doi: 10.1111/j.1467-8624.2009.01397.x [DOI] [PubMed] [Google Scholar]
- Bornstein MH, Putnick DL, Rigo P, Esposito G, Swain JE, Suwalsky JT, … & Venuti P (2017). Neurobiology of culturally common maternal responses to infant cry. Proceedings of the National Academy of Sciences, 114(45), E9465–E9473. doi: 10.1073/pnas.1712022114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borelli JL, Lai J, Smiley PA, Kerr ML, Buttitta K, Hecht HK, & Rasmussen HF (2021b). Higher maternal reflective functioning is associated with toddlers’ adaptive emotion regulation. Infant Mental Health Journal, 42(4), 473–487. doi: 10.1002/imhj.21904 [DOI] [PubMed] [Google Scholar]
- Borelli JL, Stern JA, Marvin MJ, Smiley PA, Pettit C, & Samudio M (2021a). Reflective functioning and empathy among mothers of school-aged children: Charting the space between. Emotion, 21(4), 783. doi: 10.1037/emo0000747 [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, & Carter CS (2004). Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences, 8(12), 539–546. doi: 10.1016/j.tics.2004.10.003 [DOI] [PubMed] [Google Scholar]
- Bufalari I, & Ionta S (2013). The social and personality neuroscience of empathy for pain and touch. Frontiers in Human Neuroscience, 7, 393. doi: 10.3389/fnhum.2013.00393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, & Posner MI (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6), 215–222. doi: 10.1016/S1364-6613(00)01483-2 [DOI] [PubMed] [Google Scholar]
- Carter AS, Briggs-Gowan MJ, Jones SM, & Little TD (2003). The infant–toddler social and emotional assessment (ITSEA): Factor structure, reliability, and validity. Journal of Abnormal Child Psychology, 31(5), 495–514. doi: 10.1023/a:1025449031360 [DOI] [PubMed] [Google Scholar]
- Chen YC, Jiao Y, Cui Y, Shang SA, Ding J, Feng Y, … & Teng GJ (2014). Aberrant brain functional connectivity related to insulin resistance in type 2 diabetes: a resting-state fMRI study. Diabetes Care, 37(6), 1689–1696. doi: 10.2337/dc13-2127 [DOI] [PubMed] [Google Scholar]
- Cheng Y, Chen C, Lin CP, Chou KH, & Decety J (2010). Love hurts: an fMRI study. Neuroimage, 51(2), 923–929. doi: 10.1016/j.neuroimage.2010.02.047 [DOI] [PubMed] [Google Scholar]
- Clark MA, Robertson MM, & Young S (2019). “I feel your pain”: A critical review of organizational research on empathy. Journal of Organizational Behavior, 40(2), 166–192. doi: 10.1002/job.2348 [DOI] [Google Scholar]
- Cox RW (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–173. doi: 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Davidov M, & Grusec JE (2006). Untangling the links of parental responsiveness to distress and warmth to child outcomes. Child Development, 77(1), 44–58. doi: 10.1111/j.1467-8624.2006.00855.x [DOI] [PubMed] [Google Scholar]
- Davis MH (1980). A multidimensional approach to individual differences in empathy. JSAS Catalog of Selected Documents in Psychology, 1980, 10, 85. [Google Scholar]
- Davis MH (1983). The effects of dispositional empathy on emotional reactions and helping: A multidimensional approach. Journal of Personality, 51(2), 167–184. doi: 10.1177/0886260520922345 [DOI] [Google Scholar]
- Davis MH (1996). Empathy: A social psychological approach. (1st ed.). New York, NY. doi: 10.4324/9780429493898 [DOI] [Google Scholar]
- Decety J (2015). The neural pathways, development and functions of empathy. Current Opinion in Behavioral Sciences, 3, 1–6. doi: 10.1016/j.cobeha.2014.12.001 [DOI] [Google Scholar]
- Dufford AJ, Erhart A, & Kim P (2019). Maternal brain resting-state connectivity in the postpartum period. Journal of Neuroendocrinology, 31(9), e12737. doi: 10.1111/jne.12737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, & Amunts K (2006). Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage, 32(2), 570–582. doi: 10.1016/j.neuroimage.2006.04.204 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, & Amunts K (2007). Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage, 36(3), 511–521. doi: 10.1016/j.neuroimage.2007.03.060 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, & Zilles K (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage, 25(4), 1325–1335. doi: 10.1016/j.neuroimage.2004.12.034 [DOI] [PubMed] [Google Scholar]
- Elmadih A, Wan MW, Downey D, Elliott R, Swain JE, & Abel KM (2016). Natural variation in maternal sensitivity is reflected in maternal brain responses to infant stimuli. Behavioral Neuroscience, 130(5), 500–510. doi: 10.1037/bne0000161 [DOI] [PubMed] [Google Scholar]
- Endendijk JJ, Smit AK, van Baar AL, & Bos PA (2020). What a cute baby! Preliminary evidence from a fMRI study for the association between mothers’ neural responses to infant faces and activation of the parental care system. Neuropsychologia, 143, 107493. doi: 10.1016/j.neuropsychologia.2020.107493 [DOI] [PubMed] [Google Scholar]
- Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, … & Jiang T (2016). The human brainnetome atlas: a new brain atlas based on connectional architecture. Cerebral Cortex, 26(8), 3508–3526. doi: 10.1093/cercor/bhw157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R (2015). The adaptive human parental brain: implications for children’s social development. Trends in Neurosciences, 38(6), 387–399. doi: 10.1016/j.tins.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Frith CD, & Frith U (2021). Mapping Mentalising in the Brain. In The Neural Basis of Mentalizing (pp. 17–45). doi: 10.1007/978-3-030-51890-5_2 [DOI] [Google Scholar]
- Gartstein MA, & Rothbart MK (2003). Studying infant temperament via the revised infant behavior questionnaire. Infant Behavior and Development, 26(1), 64–86. doi: 10.1016/S0163-6383(02)00169-8 [DOI] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, … & Van Essen DC (2016). A multi-modal parcellation of human cerebral cortex. Nature, 536(7615), 171–178. doi: 10.1038/nature18933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkansson J, & Montgomery H (2003). Empathy as an interpersonal phenomenon. Journal of Social and Personal Relationships, 20(3), 267–284. doi: 10.1177/0265407503020003001 [DOI] [Google Scholar]
- Ho SS, Konrath S, Brown S, & Swain JE (2014). Empathy and stress related neural responses in maternal decision making. Frontiers in Neuroscience, 8, 152. doi: 10.3389/fnins.2014.00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, & Yeung N (2012). Motivation of extended behaviors by anterior cingulate cortex. Trends in Cognitive Sciences, 16(2), 122–128. doi: 10.1016/j.tics.2011.12.008 [DOI] [PubMed] [Google Scholar]
- Humphreys KL, King LS, Choi P, & Gotlib IH (2018). Maternal depressive symptoms, self-focus, and caregiving behavior. Journal of Affective Disorders, 238, 465–471. doi: 10.1016/j.jad.2018.05.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby N, Bruneau E, Koster-Hale J, & Saxe R (2016). Localizing Pain Matrix and Theory of Mind networks with both verbal and non-verbal stimuli. Neuroimage, 126, 39–48. doi: 10.1016/j.neuroimage.2015.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joireman JA, Needham TL, & Cummings AL (2002). Relationships between dimensions of attachment and empathy. North American Journal of Psychology, 4(1), 63–80. [Google Scholar]
- Kim DH, Adalsteinsson E, Glover GH, & Spielman DM (2002). Regularized higher order in vivo shimming. Magnetic Resonance in Medicine, 48(4), 715–722. doi: 10.1002/mrm.10267 [DOI] [PubMed] [Google Scholar]
- Kim P, Feldman R, Mayes LC, Eicher V, Thompson N, Leckman JF, & Swain JE (2011). Breastfeeding, brain activation to own infant cry, and maternal sensitivity. Journal of Child Psychology and Psychiatry, 52(8), 907–915. doi: 10.1111/j.1469-7610.2011.02406.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, & Swain JE (2010). The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behavioral Neuroscience, 124(5), 695–700. doi: 10.1037/a0020884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Strathearn L, & Swain JE (2016). The maternal brain and its plasticity in humans. Hormones and Behavior, 77, 113–123. doi: 10.1016/j.yhbeh.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LS, Camacho MC, Montez DF, Humphreys KL, & Gotlib IH (2021). Naturalistic language input is associated with resting-state functional connectivity in infancy. Journal of Neuroscience, 41(3), 424–434. doi: 10.1523/JNEUROSCI.0779-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LS, Humphreys KL, Shaw GM, Stevenson DK, & Gotlib IH (2022). Validation of the assessment of parent and child adversity (APCA) in mothers and young children. Journal of Clinical Child & Adolescent Psychology, 1–16. doi: 10.1080/15374416.2022.2042696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LS, Salo VC, Kujawa A, & Humphreys KL (2021). Advancing the RDoC initiative through the assessment of caregiver social processes. Development and Psychopathology, 33(5), 1648–1664. doi: 10.1017/S095457942100064X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight LK, Stoica T, Fogleman ND, & Depue BE (2019). Convergent neural correlates of empathy and anxiety during socioemotional processing. Frontiers in Human Neuroscience, 13, 94. doi: 10.3389/fnhum.2019.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G, Friesenborg AE, Lange LA, & Martel MM (2004). Parents’ personality and infants’ temperament as contributors to their emerging relationship. Journal of Personality and Social Psychology, 86(5), 744–759. doi: 10.1037/0022-3514.86.5.744 [DOI] [PubMed] [Google Scholar]
- Kostyrka-Allchorne K, Wass SV, & Sonuga-Barke EJ (2020). Research Review: Do parent ratings of infant negative emotionality and self-regulation predict psychopathology in childhood and adolescence? A systematic review and meta-analysis of prospective longitudinal studies. Journal of Child Psychology and Psychiatry, 61(4), 401–416. doi: 10.1111/jcpp.13144 [DOI] [PubMed] [Google Scholar]
- Leerkes EM (2010). Predictors of maternal sensitivity to infant distress. Parenting: Science and Practice, 10(3), 219–239. doi: 10.1080/15295190903290840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leerkes EM, Blankson AN, & O’Brien M (2009). Differential effects of maternal sensitivity to infant distress and nondistress on social-emotional functioning. Child Development, 80(3), 762–775. doi: 10.1111/j.1467-8624.2009.01296.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzi D, Trentini C, Pantano P, Macaluso E, Iacoboni M, Lenzi GL, & Ammaniti M (2009). Neural basis of maternal communication and emotional expression processing during infant preverbal stage. Cerebral Cortex, 19(5), 1124–1133. doi: 10.1093/cercor/bhn153 [DOI] [PubMed] [Google Scholar]
- Levin HS, Williams DH, Eisenberg HM, High WM, & Guinto FC (1992). Serial MRI and neurobehavioural findings after mild to moderate closed head injury. Journal of Neurology, Neurosurgery & Psychiatry, 55(4), 255–262. doi: 10.1136/jnnp.55.4.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Cao W, Zhang X, Sun B, Jiang S, Li J, … & Luo, C. (2019). BOLD-fMRI reveals the association between renal oxygenation and functional connectivity in the aging brain. NeuroImage, 186, 510–517. doi: 10.1016/j.neuroimage.2018.11.030 [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang T, Li W, Zhang J, Jin Z, & Li L (2020). Linking brain structure and activation in anterior insula cortex to explain the trait empathy for pain. Human Brain Mapping, 41(4), 1030–1042. doi: 10.1002/hbm.24858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd D, Di Pellegrino G, & Roberts N (2004). Vicarious responses to pain in anterior cingulate cortex: is empathy a multisensory issue?. Cognitive, Affective, & Behavioral Neuroscience, 4(2), 270–278. doi: 10.3758/cabn.4.2.270 [DOI] [PubMed] [Google Scholar]
- Lockwood PL (2016). The anatomy of empathy: Vicarious experience and disorders of social cognition. Behavioural Brain Research, 311, 255–266. doi: 10.1016/j.bbr.2016.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood PL, Apps MA, Roiser JP, & Viding E (2015). Encoding of vicarious reward prediction in anterior cingulate cortex and relationship with trait empathy. Journal of Neuroscience, 35(40), 13720–13727. doi: 10.1523/JNEUROSCI.1703-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Robinson JL, Glahn DC, & Fox PT (2010). Acute effects of hydrocortisone on the human brain: an fMRI study. Psychoneuroendocrinology, 35(1), 15–20. doi: 10.1016/j.psyneuen.2009.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manczak EM, DeLongis A, & Chen E (2016). Does empathy have a cost? Diverging psychological and physiological effects within families. Health Psychology, 35(3), 211–218. doi: 10.1037/hea0000281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Fowler KA, Adalio CJ, Jurkowitz IT, Schechter JC, … & Blair RJR (2013). Empathic responsiveness in amygdala and anterior cingulate cortex in youths with psychopathic traits. Journal of Child Psychology and Psychiatry, 54(8), 900–910. doi: 10.1111/jcpp.12063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin R, Cooper G, Hoffman K, & Powell B (2002). The Circle of Security project: Attachment-based intervention with caregiver-pre-school child dyads. Attachment & Human Development, 4(1), 107–124. doi: 10.1080/14616730210131635 [DOI] [PubMed] [Google Scholar]
- Masten CL, Morelli SA, & Eisenberger NI (2011). An fMRI investigation of empathy for ‘social pain’ and subsequent prosocial behavior. NeuroImage, 55(1), 381–388. doi: 10.1016/j.neuroimage.2010.11.060 [DOI] [PubMed] [Google Scholar]
- Meins E, Centifanti LCM, Fernyhough C, & Fishburn S (2013). Maternal mind mindedness and children’s behavioral difficulties: Mitigating the impact of low socioeconomic status. Journal of Abnormal Child Psychology, 41(4), 543–553. doi: 10.1007/s10802-012-9699-3 [DOI] [PubMed] [Google Scholar]
- Meins E, Fernyhough C, Fradley E, & Tuckey M (2001). Rethinking maternal sensitivity: Mothers’ comments on infants’ mental processes predict security of attachment at 12 months. The Journal of Child Psychology and Psychiatry and Allied Disciplines, 42(5), 637–648. doi: 10.1111/1469-7610.00759 [DOI] [PubMed] [Google Scholar]
- Meins E, Fernyhough C, Wainwright R, Clark-Carter D, Das Gupta M, Fradley E, & Tuckey M (2003). Pathways to understanding mind: Construct validity and predictive validity of maternal mind-mindedness. Child Development, 74(4), 1194–1211. doi: 10.1111/1467-8624.00601 [DOI] [PubMed] [Google Scholar]
- Menon V, & Uddin LQ (2010). Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function, 214(5–6), 655–667. doi: 10.1007/s00429-010-0262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JG, Kahle S, Lopez M, & Hastings PD (2015). Compassionate love buffers stress reactive mothers from fight-or-flight parenting. Developmental Psychology, 51(1), 36–43. doi: 10.1037/a0038236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JG, Xia G, & Hastings PD (2020). Right temporoparietal junction involvement in autonomic responses to the suffering of others: A preliminary transcranial magnetic stimulation study. Frontiers in Human Neuroscience, 14, 7. doi: 10.3389/fnhum.2020.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezlek JB, Feist GJ, Wilson FC, & Plesko RM (2001). Day-to-day variability in empathy as a function of daily events and mood. Journal of Research in Personality, 35(4), 401–423. doi: 10.1006/jrpe.2001.2332 [DOI] [Google Scholar]
- Oppenheim D, & Koren-Karie N (2002). Mothers’ insightfulness regarding their children’s internal worlds: The capacity underlying secure child–mother relationships. Infant Mental Health Journal: Official Publication of The World Association for Infant Mental Health, 23(6), 593–605. doi: 10.1002/imhj.10035 [DOI] [Google Scholar]
- Oppenheim D, Koren-Karie N, & Sagi A (2001). Mothers’ empathic understanding of their preschoolers’ internal experience: Relations with early attachment. International journal of Behavioral Development, 25(1), 16–26. doi: 10.1080/01650250042000096 [DOI] [Google Scholar]
- Pak RW, Hadjiabadi DH, Senarathna J, Agarwal S, Thakor NV, Pillai JJ, & Pathak AP (2017). Implications of neurovascular uncoupling in functional magnetic resonance imaging (fMRI) of brain tumors. Journal of Cerebral Blood Flow & Metabolism, 37(11), 3475–3487. doi: 10.1177/0271678X17707398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorelli C, Lansford JE, Luengo Kanacri BP, Malone PS, Di Giunta L, Bacchini D, … & Sorbring E (2016). Positive parenting and children’s prosocial behavior in eight countries. Journal of Child Psychology and Psychiatry, 57(7), 824–834. doi: 10.1111/jcpp.12477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Albeniz A, & de Paul J (2003). Dispositional empathy in high-and low-risk parents for child physical abuse. Child Abuse & Neglect, 27(7), 769–780. doi: 10.1016/s0145-2134(03)00111-x [DOI] [PubMed] [Google Scholar]
- Preckel K, Kanske P, & Singer T (2018). On the interaction of social affect and cognition: empathy, compassion and theory of mind. Current Opinion in Behavioral Sciences, 19, 1–6. doi: 10.1016/j.cobeha.2017.07.010 [DOI] [Google Scholar]
- Putnam SP, Helbig AL, Gartstein MA, Rothbart MK, & Leerkes E (2014). Development and assessment of short and very short forms of the Infant Behavior Questionnaire–Revised. Journal of Personality Assessment, 96(4), 445–458. doi: 10.1080/00223891.2013.841171 [DOI] [PubMed] [Google Scholar]
- Rameson LT, Morelli SA, & Lieberman MD (2012). The neural correlates of empathy: experience, automaticity, and prosocial behavior. Journal of Cognitive Neuroscience, 24(1), 235–245. doi: 10.1162/jocn_a_00130 [DOI] [PubMed] [Google Scholar]
- Reindl V, Gerloff C, Scharke W, & Konrad K (2018). Brain-to-brain synchrony in parent child dyads and the relationship with emotion regulation revealed by fNIRS-based hyperscanning. NeuroImage, 178, 493–502. doi: 10.1016/j.neuroimage.2018.05.060 [DOI] [PubMed] [Google Scholar]
- Reynolds WJ, & Scott B (1999). Empathy: a crucial component of the helping relationship. Journal of Psychiatric and Mental Health Nursing, 6(5), 363–370. doi: 10.1046/j.1365-2850.1999.00228.x [DOI] [PubMed] [Google Scholar]
- Roca J, Grau M, & Balasch J (1981). Effect of pirozadil on cerebral blood flow in anesthetized dogs. Methods and Findings in Experimental and Clinical Pharmacology, 3(6), 397–401. [PubMed] [Google Scholar]
- Rosseel Y (2012). lavaan: An R package for structural equation modeling. Journal of Statistical Software, 48, 1–36. doi: 10.18637/jss.v048.i02 [DOI] [Google Scholar]
- Salo VC, Schunck SJ, & Humphreys KL (2020). Depressive symptoms in parents are associated with reduced empathy toward their young children. PLoS One, 15(3), e0230636. doi: 10.1371/journal.pone.0230636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, & Powell LJ (2006). It’s the thought that counts: specific brain regions for one component of theory of mind. Psychological Science, 17(8), 692–699. doi: 10.1111/j.1467-9280.2006.01768.x [DOI] [PubMed] [Google Scholar]
- Schleim S, & Roiser JP (2009). fMRI in translation: the challenges facing real-world’ applications. Frontiers in Human Neuroscience, 3, 63. doi: 10.3389/neuro.09.063.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz M, Radua J, Aichhorn M, Richlan F, & Perner J (2014). Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neuroscience & Biobehavioral Reviews, 42, 9–34. doi: 10.1016/j.neubiorev.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’doherty J, Kaube H, Dolan RJ, & Frith CD (2004). Empathy for pain involves the affective but not sensory components of pain. Science, 303(5661), 1157–1162. doi: 10.1126/science.1093535 [DOI] [PubMed] [Google Scholar]
- Stern JA, Borelli JL, & Smiley PA (2015). Assessing parental empathy: A role for empathy in child attachment. Attachment & Human Development, 17(1), 1–22. doi: 10.1080/14616734.2014.969749 [DOI] [PubMed] [Google Scholar]
- Suchman NE, DeCoste C, Castiglioni N, McMahon TJ, Rounsaville B, & Mayes L (2010). The Mothers and Toddlers Program, an attachment-based parenting intervention for substance using women: Post-treatment results from a randomized clinical pilot. Attachment & Human Development, 12(5), 483–504. doi: 10.1002/imhj.20303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchman NE, DeCoste CL, McMahon TJ, Dalton R, Mayes LC, & Borelli J (2017). Mothering From the Inside Out: Results of a second randomized clinical trial testing a mentalization-based intervention for mothers in addiction treatment. Development and Psychopathology, 29(2), 617–636. doi: 10.1017/S0954579417000220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE (2011). The human parental brain: in vivo neuroimaging. Progress in Neuro Psychopharmacology and Biological Psychiatry, 35(5), 1242–1254. doi: 10.1016/j.pnpbp.2010.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons DK, & Clark SE (2000). A longitudinal study of mother-child relationships and theory of mind in the preschool period. Social Development, 9(1), 3–23. doi: 10.1111/1467-9507.00108 [DOI] [Google Scholar]
- Tan Q, Zhan Y, Gao S, Fan W, Chen J, & Zhong Y (2015). Closer the relatives are, more intimate and similar we are: kinship effects on self-other overlap. Personality and Individual Differences, 73, 7–11. doi: 10.1016/j.paid.2014.08.038 [DOI] [Google Scholar]
- Tang Y, Meng LI, Wan CM, Liu ZH, Liao WH, Yan XX, … & Guo, J. F. (2017). Identifying the presence of Parkinson’s disease using low-frequency fluctuations in BOLD signals. Neuroscience Letters, 645, 1–6. doi: 10.1016/j.neulet.2017.02.056 [DOI] [PubMed] [Google Scholar]
- Team R. Core (2020). R: A language and environment for statistical computing, 3(3). [Google Scholar]
- Upshaw MB, Kaiser CR, & Sommerville JA (2015). Parents’ empathic perspective taking and altruistic behavior predicts infants’ arousal to others’ emotions. Frontiers in Psychology, 6, 360. doi: 10.3389/fpsyg.2015.00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boom DC (1994). The relationship between attachment, temperament, and exploration. In Curiosity and Exploration (pp. 123–150). Springer, Berlin, Heidelberg. doi: 10.1007/978-3-642-77132-3_8 [DOI] [Google Scholar]
- Veldsman M, Cumming T, & Brodtmann A (2015). Beyond BOLD: optimizing functional imaging in stroke populations. Human Brain Mapping, 36(4), 1620–1636. doi: 10.1002/hbm.22711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP, Iacoboni M, & Mazziotta JC (1994). Bilateral spreading cerebral hypoperfusion during spontaneous migraine headache. New England Journal of Medicine, 331(25), 1689–1692. doi: 10.1056/NEJM199412223312505 [DOI] [PubMed] [Google Scholar]
- Xia W, Rao H, Spaeth AM, Huang R, Tian S, Cai R, … & Wang S (2015). Blood pressure is associated with cerebral blood flow alterations in patients with T2DM as revealed by perfusion functional MRI. Medicine, 94(48), e2231. doi: 10.1097/MD.0000000000002231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HC, Liang Z, Yao J, Shen X, Frederick BD, & Tong Y (2019). Vascular effects of caffeine found in BOLD fMRI. Journal of Neuroscience Research, 97(4), 456–466. doi: 10.1002/jnr.24360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KS, Parsons CE, Stein A, Vuust P, Craske MG, & Kringelbach ML (2017). The neural basis of responsive caregiving behaviour: Investigating temporal dynamics within the parental brain. Behavioural Brain Research, 325, 105–116. doi: 10.1016/j.bbr.2016.09.012 [DOI] [PubMed] [Google Scholar]
- Zafiris O, Kircheis G, Rood HA, Boers F, Häussinger D, & Zilles K (2004). Neural mechanism underlying impaired visual judgement in the dysmetabolic brain: an fMRI study. NeuroImage, 22(2), 541–552. doi: 10.1016/j.neuroimage.2004.01.038 [DOI] [PubMed] [Google Scholar]
- Zaki J (2014). Empathy: a motivated account. Psychological Bulletin, 140(6), 1608–1647. doi: 10.1037/a0037679 [DOI] [PubMed] [Google Scholar]
- Zaki J, & Ochsner KN (2012). The neuroscience of empathy: progress, pitfalls and promise. Nature Neuroscience, 15(5), 675–680. doi: 10.1038/nn.3085 [DOI] [PubMed] [Google Scholar]
- Zhang K, Rigo P, Su X, Wang M, Chen Z, Esposito G, … & Du X (2020). Brain responses to emotional infant faces in new mothers and nulliparous women. Scientific Reports, 10(1), 1–10. doi: 10.1038/s41598-020-66511-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Eisenberg N, Losoya SH, Fabes RA, Reiser M, Guthrie IK, … & Shepard SA (2002). The relations of parental warmth and positive expressiveness to children’s empathy-related responding and social functioning: A longitudinal study. Child Development, 73(3), 893–915. doi: 10.1111/1467-8624.00446 [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, & Carmignoto G (2003). Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nature Neuroscience, 6(1), 43–50. doi: 10.1038/nn980 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed in the current study will be made available by the corresponding author upon reasonable request.


