Abstract

This study reveals the state-of-the-art fabrication of a tripolymer-based electrospun nanofiber (NF) system to enhance the release, solubility, and transdermal penetration of curcumin (Cur) with the aid of in situ release of infused castor oil (Co). In this regard, Cur-loaded Co-infused polyethylene oxide (PEO), ethyl cellulose (EC), and polyvinyl pyrrolidone (PVP) tripolymer-based NF systems were developed to produce a hybridized transdermal skin patch. Weight percentages of 1–4% Cur and 3–10% of Co were blended with PEO–EC–PEO and PEO–EC–PVP polymer systems. The prepared NFs were characterized by SEM, TEM, FT-IR analysis, PXRD, differential scanning calorimetry (DSC), and XPS. Dialysis membranes and vertical Franz diffusion cells were used to study the in vitro drug release and transdermal penetration, respectively. The results indicated that maintaining a Cur concentration of 1–3 wt % with 3 wt % Co in both PEO–EC–Co–Cur@PEO and PEO–EC–Co–Cur@PVP gave rise to nanofibers with lowered diameters (144.83 ± 48.05–209.26 ± 41.80 nm and 190.20 ± 59.42–404.59 ± 45.31 nm). Lowered crystallinity observed from the PXRD patterns and the disappearance of exothermic peaks corresponding to the melting point of Cur suggested the formation of an amorphous NF structure. Furthermore, the XPS data revealed that the Cur loading will possibly take place at the inner interface of PEO–EC–Co–PEO and PEO–EC–Co–PVP NFs rather than on the surface. The beneficiary role of Co on the release and dermal penetration of Cur was further confirmed from the respective release data which indicated that PEO–EC–Co–Cur@PEO would lead to a rapid release (4–5 h), while PEO–EC–Co–Cur@PVP would lead to a sustained release over a period of 24 h in the presence of Co. Transdermal penetration of the released Cur was further evidenced with the development of color in the receiver compartment of the diffusion cell. DPPH results further corroborated that a sustained antioxidant activity is observed in the released Cur where the free-radical scavenging activity is intact even after subjecting to an electrospinning process and under extreme freeze–thaw conditions.
1. Introduction
Curcumin, one of the major curcuminoids of the turmeric extract, is among the most beneficial herbs known for long-lasting therapeutic healing for thousands of years.1 It is proven that curcumin’s antioxidant and anti-inflammatory properties could help maintain a better cognition and mobility to cardiometabolic and gut health.2 It is also considered as a natural pain and stress reliever and is successful as a medication for diabetes, fatigue, and rheumatoid arthritis.3 It acts as a master switch in inflammation by downregulating the expression of genes corresponding to many proinflammatory enzymes, transcription factors, and inflammatory cytokines.4 In addition, curcumin has also been considered as a nontoxic, safe, and freely available antibiotic for antimicrobial resistance which is one of the biggest challenges facing the world.5
It is also a well-known ingredient in cosmetic products due to its healing and regenerative power.6 More importantly, its contribution toward various skin disorders is massive. In many instances, curcumin acts as a free-radical quencher, reduces inflammation by inhibiting the nuclear factor NF-κB, reduces excessive production of proinflammatory cytokines, activates tumor suppressor genes, and aids in wound healing by triggering collagen deposition and fibroblast formation and inducing growth factors involved in angiogenesis as well as extracellular matrix formation.7 Because it is widely available and well tolerated while providing a pleiotropic effect, it has been effective in treating eczema, psoriasis, scleroderma, and skin cancer.8 Because of the specified beneficial roles of curcumin, it is also one of the fastest growing dietary supplements with increased sales, sharing the limelight in the market.9
However, the biggest challenge of the clinical application of curcumin as a dietary supplement is its poor bioavailability. Because of its lipophilic nature, its solubility and absorption in aqueous media have become limited, leading to a poor pharmacokinetic profile.10−12 In addition, extreme light and pH sensitivities of curcumin also reduced the actual clinical application.13 Furthermore, curcumin is also susceptible to first-pass metabolism, leading to the production of metabolites with less potency.7
In this regard, topical administration of curcumin led to improved bioavailability due to the easy accessibility of curcumin at the localized sites.14 In many of these localized delivery approaches, curcumin has been trapped inside a carrier, from which its release has taken place. Various delivery platforms such as microcapsules,15 multilayered microcarriers,16 hydrogels,17 polymeric bandages,18 nanoemulsions,19 sponges,20 and nanoparticulate systems21 have been exploited over the application of curcumin for disease purposes. Generally, the release of curcumin from these drug carriers is not a spontaneous process because of its inherent poor aqueous solubility. Specially during the topical application of curcumin, an excipient that improves its release as well as solubility will be essential to allow its absorption and subsequent permeable delivery. Therefore, the use of additives like beta-cyclodextrin (β-CD)22 and amino acids,23 application of a mechanical force (e.g., rubbing),24 or the use of a releasing medium that contains solubilization enhancers like polysorbate (Tween 80)25,26 has always been the choice for this purpose.
Therefore, the development of a skin patch having a dual performance ability of inherent curcumin release (in the presence of moisture or sweat as the stimuli) along with the enhanced skin permeability will be a novel approach to improve the bioavailability of curcumin targeted for skin applications. Fabrication of skin patches involves various practices such as the use of adhesive- or reservoir-based diffusion system having single or multiple layers,27 matrix-based systems,28 microneedles/microarray patches,29 and electrospun nanofibrous mats, out of which electrospinning, being a versatile and economic technique that produces nanofibers, has received a greater deal of attention in recent years.30,31 Electrospun nanofibers have many attractive properties such as large surface-to-volume ratio that provides efficient drug delivery, specialized architecture that triggers controlled release, and flexible nature that makes them ideal for topical application.32
Topical delivery of curcumin via polymer-based electrospun nanofibers has also been investigated for various diseases such as psoriasis,33 atopic dermatitis,34 acne,35 skin cancer,36 wound care,37 and in skin infections.38 However, the use of state-of-the-art tripolymer-based electrospun system fused with polyethylene oxide (PEO), ethyl cellulose (EC), and polyvinyl pyrrolidone (PVP) polymers leading to the development of a skin patch for curcumin release will be proposed in this study. More particularly, this study is aimed to produce self-triggered, curcumin-releasing, solubilizing, and improved permeable system for the effective delivery of curcumin through the skin. For this purpose, PEO 40 hydrogenated castor oil (Co), a widely used solubilizer in skin care products39 will be blended along with the polymer compounds to improve curcumin’s solubility. Furthermore, it is assumed that the conceptual design of a tripolymer-blended nanofiber structure would compartmentalize curcumin from castor oil, thereby allowing the simultaneous release of both compounds while triggering the solubility as well as absorption/penetration of released curcumin. This approach of using self-driven curcumin-releasing electrospun fibrous mat will be useful in developing adhesive skin patches to release curcumin for wound exudates or to produce innovative smart fabric inserts for active wear which will be helpful in designing disease-preventive medical textiles targeting their application in major diseases such as cancer (skin or breast cancer).
2. Results and Discussion
2.1. Preparation and Morphology of Curcumin-Loaded Nanofibers
After the careful observation of the previous literature, a very few works have involved in the preparation of PEO–EC nanofiber blends for specific reasons. According to the work suggested by Huang,40 ethyl cellulose, polyethylene oxide, and cellulose acetate nonwovens have been used to encapsulate thymol and carvacrol. Adapting these procedures which have been used to make the polymer blend, 80% ethanol was used in our study to solubilize the PEO and EC polymer molecules. After the optimization of the process parameters (data not given), the final polymer ratio of EC:PEO was fixed at 4:1 that accounts for a total polymer concentration of 2% (w/v) to obtain smoother and thinner fibers. EC is known to be a hydrophobic polymer,41 whereas PEO is known to be hydrophilic.42 The loading of curcumin into the PEO–EC nanofiber blend would lead to a better encapsulation of curcumin, but still this system will lack a component that improves the release, dissolution, and the skin penetration of the infused curcumin. This highlights the necessity of Co. Even though there are multiple studies involved in the synthesis of curcumin nanofibers for various purposes,43−47 this will be the first ever detailed study that reports the use of a tripolymer-based system (PEO, EC, and PVP) combined with hydrogenated castor oil to improve the real applicability of released curcumin for any skin-based application.
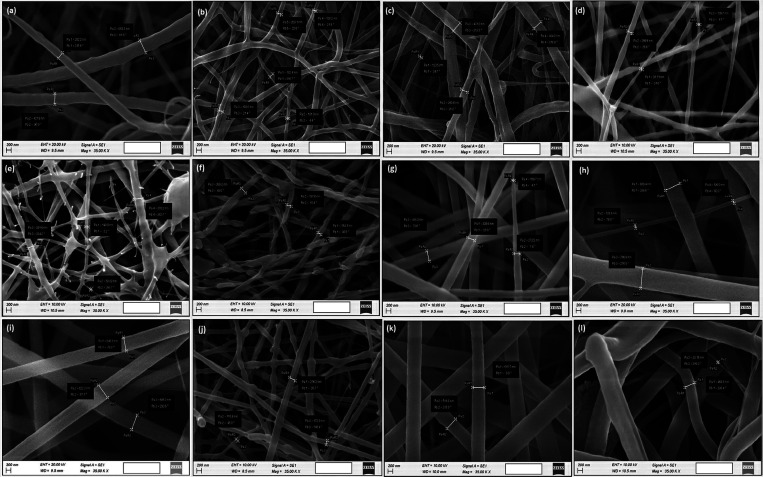
Figure 1 depicts the characteristic morphological features of the prepared nanofibers with different compositions of PEO, EC, PVP, curcumin, and Co. Further, Table 1 indicates the mean fiber diameter ± SD (nm) of the resulted fibers. Considering the PEO–EC–Co–Cur@PEO nanofiber system, it is evidenced that with the increase of curcumin (at a constant amount of Co), the cross-linking as well as the fiber diameter of fibers increased (for 3 and 4 wt % curcumin-added systems). However, at a very low concentration of curcumin (1 wt %), the nanofibers are polydispersed, accounting for the highest average diameter (367.49 ± 98.38 nm), but it led to much smoother nanofibers compared to the ones formed at higher curcumin concentrations (3 and 4 wt % curcumin-added systems). When considering the effect of Co (at a constant amount of curcumin), with the increase of Co concentration from 3 to 10 wt %, the fibers tend to get larger (144.83 ± 48.05 nm, 186.21 ± 114.06 nm, and 209.26 ± 41.80), but overall, the increase of Co has positively contributed to the reduction of nanofiber size compared to the systems with increased curcumin concentration. However, the excessive increase of Co amount (more than 5 wt %) has negatively impacted the fiber morphology, leading to more beading of the fibers and fiber-breaking, suggesting the occurrence of instability.
Figure 1.
Size distribution of nanofibers that resulted from PEO-EC-Co-Cur@PEO and PEO-EC-Co-Cur@PVP systems. (a) PEO-EC-Co(0.2%)-Cur(1%)@PEO, (b) PEO-EC-Co(0.2%)-Cur(3%)@PEO, (c) PEO-EC-Co(0.2%)-Cur(4%)@PEO, (d) PEO-EC-Co(3%)-Cur(1%)@PEO, (e)PEO-EC-Co(5%)-Cur(1%)@PEO, (f) PEO-EC-Co(10%)-Cur(1%)@PEO, (g)PEO-EC-Co(0.2%)-Cur(1%)@PVP, (h)PEO-EC-Co(0.2%)-Cur(3%)@PVP, (i) PEO-EC-Co(0.2%)-Cur(4%)@PVP, (j) PEO-EC-Co(3%)-Cur(1%)@PVP, (k) PEO-EC-Co(5%)-Cur(1%)@PVP, and (l) PEO-EC-Co(10%)-Cur(1%)@PVP.
Table 1. Fiber Diameters of the Nanofibers Resulted from the Blending of Curcumin and Hydrogenated Castor Oil.
| name of the system | mean fiber diameter ± SD (nm) |
|---|---|
| PEO-EC-Co(0.2%)-Cur(1%)@PEO | 367.49 ± 98.38 |
| PEO-EC-Co(0.2%)-Cur(3%)@PEO | 164.22 ± 37.78 |
| PEO-EC-Co(0.2%)-Cur(4%)@PEO | 313.40 ± 96.00 |
| PEO-EC-Co(3%)-Cur(1%)@PEO | 144.83 ± 48.05 |
| PEO-EC-Co(5%)-Cur(1%)@PEO | 186.21 ± 114.06 |
| PEO-EC-Co(10%)-Cur(1%)@PEO | 209.26 ± 41.80 |
| PEO-EC-Co(0.2%)-Cur(1%)@PVP | 317.21 ± 79.97 |
| PEO-EC-Co(0.2%)-Cur(3%)@PVP | 581.27 ± 222.44 |
| PEO-EC-Co(0.2%)-Cur(4%)@PVP | 588.92 ± 26.88 |
| PEO-EC-Co(3%)-Cur(1%)@PVP | 190.20 ± 59.42 |
| PEO-EC-Co(5%)-Cur(1%)@PVP | 526.44 ± 12.89 |
| PEO-EC-Co(10%)-Cur(1%)@PVP | 404.59 ± 45.31 |
Similarly, in PEO–EC–Co–Cur@PVP nanofibrous systems, increase of the curcumin concentration from 1 to 4 wt % has also led to the increase of the average fiber diameter (317.21 ± 79.97, 581.27 ± 222.44, and 588.92 ± 26.88 nm), but they tend to appear quite different to the PEO–EC–Co–Cur@PEO nanofiber system. More importantly, fibers become homogeneous with the increase of the curcumin content with no cross-linking. Likewise, the increase of Co concentration in PEO–EC–Co–Cur@PVP at a constant amount of curcumin again led to the increase of the average fiber diameter in each system of consideration (3, 5, and 10%), but the increase of Co content resulted in the overall reduction of fiber diameter compared to the effect created by the increase of the curcumin content. Moreover, the resultant fibers are much smoother in appearance than the PEO-based system. In general, in both PEO–EC–Co–Cur@PEO and PEO–EC–Co–Cur@PVP systems, the increase of curcumin resulted in the increase of the overall fiber diameter in contrast to the observed results with the increase of Co content. Further, the resultant nanofibers of the PEO–EC–Co–Cur@PVP system are much larger than the nanofibers of the PEO–EC–Co–Cur@PEO system.
The increase of the fiber diameter with the increased addition of curcumin, both in PEO–EC–Co–Cur@PEO and PEO–EC-Co-Cur@PVP, could be due to the increased loading of the curcumin content. Furthermore, this increase is much prominent in PEO–EC–Co–Cur@PVP nanofibers due to the higher weight percentage of PVP triggering the chain entanglement and the possible favorable interactions between the PVP and curcumin molecules.48 PVP contains a carbon backbone to which pyrrolidone rings are attached. It is reported that the hydrogen bonding would take place in between the hydroxyl group of the phenol ring of curcumin and the carbonyl oxygen of PVP. With regard to PEO, it is reported that the hydrogen bonds are generally formed in between the end hydroxyl group of PEO and the phenol hydroxyl group of curcumin, being more labile to break due to the long polymer chain.49 Also, by considering the structure and the possible stacking of PVP and PEO molecules, it could be assumed that PVP molecules would stack much more favorably49 in between the curcumin molecules than with the PEO molecules, thereby leading to better interactions resulting with the increased fiber diameter. Overall, maintaining a curcumin concentration of 1–3 wt % with 3 wt % Co in both PEO–EC–Co–Cur@PEO and PEO–EC–Co–Cur@PVP gave rise to nanofibers with lowered diameter.
The TEM images of PEO–EC–Co–Cur@PEO and PEO–EC–Co–Cur@PVP systems are given in Figure S1. It is very clear that the PEO–EC–Co–Cur@PEO system (Figure S1a,b) leads to a branched nanofibrous structure, whereas the PEO–EC–Co–Cur@PVP system results in much bigger fibers while maintaining monodispersity (Figure S1c,d). Further, the TEM images indicate a clear demarcation between two phases in the nanofiber structure which could evidence an internal core and an outer covering. The internal core structure could be due to the loading of the curcumin molecules, as observed in previous studies.50 Moreover, the internal core of PEO–EC–Co–Cur@PVP (Figure S1b) is much larger compared to that of the PEO–EC–Co–Cur@PEO system (Figure S1d).
2.2. FT-IR Analysis
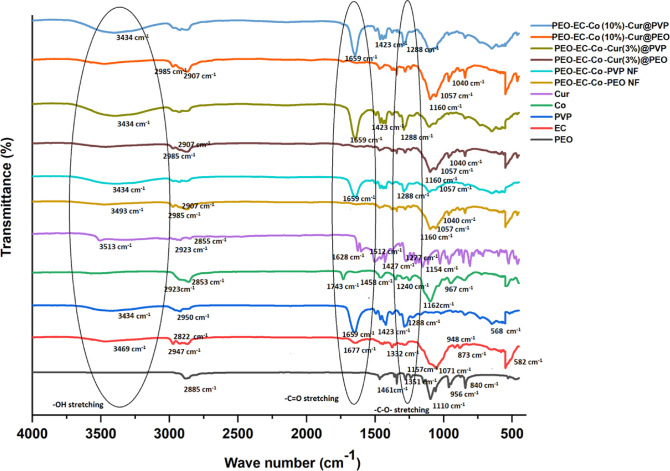
The FT-IR spectra in Figure 2 shows the characteristic peaks of the original compounds, PEO-EC-Co-PEO, PEO-EC-Co-PVP and PEO-EC-Co-Cur@PEO and PEO-EC-Co-Cur@PVP NFs. The vibrational bands of the neat polymer PEO appearing at 2885, 1461, 1345, 1110—, 956 and 840 cm–1 due to C–H asymmetric stretching, −CH2 scissoring, −CH2 asymmetric bending, −C–O–C– stretching, −CH2 asymmetric stretching, and −C–O– stretching, respectively,51 also appeared in the PEO-EC-Co-PEO NF while showing some overlaps with the characteristic peaks of EC and Co compounds.52,53 However, compared with the characteristic peaks of the neat PEO polymer, the peaks appearing in the blended fiber due to the PEO vibrational bands are clearly visible with reduced peak intensity (Figures 2 and S2), as observed in similar studies.54 The vibrational spectrum of the PEO-EC-Co-PEO NF resembles more of the vibrational pattern of the neat EC polymer, along with the appearance of bands corresponding to both PEO and Co. The bands due to −OH stretching vibrations at 3493 cm–1, −C–H– stretching vibrations at 2907 and 2985 cm–1, as well as −C–O–C– stretching and −C–H– bending vibrations at 1057 and 1379 cm–152 could be found in the blended fiber of PEO-EC-Co-PEO. The intensity of the −OH stretching band of neat EC reduced when blended into the PEO-EC-Co-PEO polymer system while giving rise to a much broader −OH stretching vibration in the blended fiber, highlighting that the possible hydrogen bonding55 between the hydroxyl groups and carbonyl appear in PEO and Co, respectively. The peaks that could arise due to the Co polymer are not clearly distinguishable in the blended fiber, which could be possible due to the overlapping regions with both PEO and EC.
Figure 2.
FT-IR spectra of PEO, EC, PVP, Co, Cur, PEO-EC-Co-PEO NF, PEO-EC-Co-PVP-NF, PEO-EC-Co-Cur(3%)@PEO NF, PEO-EC-Co-Cur(3%)@PVP NF, PEO-EC-Co(10%)-Cur@PEO, and PEO-EC-Co(10%)-Cur@PVP.
When considering the PEO-EC-Co-PVP NF system, it is clear that the vibrational pattern represents more of the pattern of neat PVP polymer with reduced intensity, whereas the effect coming from PEO, EC, and Co is heavily masked due to the possible overlapping regions of the vibrational bands (Figures 2 and S3). The carbonyl vibration of PVP at 1659 cm–1 decreased and became broader with the blending of PVP with the PEO-EC-Co system.55 In addition, clear absorption peaks corresponding to the −C–H symmetric (2950 cm–1) and −O–H symmetric stretching vibrations (3434 cm–1) of the PVP polymer backbone56 can be found in the PEO-EC-Co-PVP blended fiber. The vibrational peaks appearing in the region of 1000–1250 cm–1 of PEO-EC-Co-PVP could appear due to the −C–O–C– stretching (1057 cm–1) of EC and Co, bending of -CH2 groups (1235 cm–1) of EC and Co, as well as the bending vibrations of carbonyl groups of Co (1160–1040 cm–1).52,53
It can be clearly identified that the blending of curcumin with the PEO-EC-Co-PEO polymer system led to the disappearance or faint appearance of the characteristic bands of curcumin such as the carbonyl bond vibration at 1628 cm–1, −O–H stretching vibrations at 3300–3500 cm–1, −C=C– aromatic stretching vibration at 1427 cm–1, in-plane bending vibrations at 1512 cm–1, and aromatic −C=O– stretching and −C–O–C stretching bands at 1277 and 1154 cm–1, respectively.57,58 The inability to clearly identify the bands corresponding to curcumin could possibly arise due to the overlapping of the said vibrational bands with that of PEO, EC, and Co, as well as the encapsulation of curcumin taking place inside the nanofiber system, leading to a very less amount of curcumin appearing on the surface. Further, the absence of phenolic −O–H stretching of curcumin will indicate the possible H bonding with the hydroxyl groups of PEO and carbonyl group of Co.57
Similarly, in the situation where curcumin is blended with the PEO-EC-Co-PVP polymer system (Figures 2 and S3), the phenolic −OH of curcumin must have contributed to the formation of hydrogen bonding with PEO-EC-Co-PVP, as the intensity of the −O–H band in PEO-EC-Co-Cur@PVP is higher than that of PVP or PEO-EC-Co-PVP NF system, as observed in another study.48 Considering the PEO-EC-Co-Cur@PVP NF systems, the vibrational patterns resemble more of PVP > Co > EC > Cur, highlighting the possibility that the diverse compounds with similar organic functional groups could absorb at a similar spectral range. The absence of characteristic bands of curcumin in PEO-EC-Co-Cur@PVP may indicate the PEO-EC-Co-PVP polymer blend as a good carrier for the encapsulation of curcumin molecules. Favorable hydrogen bonding between curcumin and PVP is further proven with the disappearance of the sharp peak of phenolic stretching of curcumin and the shift of carbonyl stretching vibration of PVP into a lower wavenumber.59
2.3. XRD Analysis of the NF Systems
The XRD spectral patterns (Figure 3) of pristine curcumin and PEO show sharp peaks at 8.6, 11.95, 14.38, 17.02, 18, 20.78, 23.08, and 29.26 corresponding to curcumin60 and two intense peaks at 19.2° and 23.6° due to the semicrystalline nature of PEO. PVP being amorphous showed only two broad peaks at 11° and 22°, respectively.61 Neat EC showed one characteristic and another broad peak at 2θ equal to 11.1° and 20.3°.62 However, when the composite nanofibers PEO-EC-Co-PEO and PEO-EC-Co-PVP are formed, it is clear that the blending of polymers affected the orientation of polymer molecules to produce a nanofibrous structure that increased the amorphous nature of the polymers, leading to the reduction of crystallinity, as observed in similar studies.55 The underlying reason for such a reduction of crystallinity could be due to the electrospinning process that leads to rapid solvent evaporation, allowing a short traveling time before the deposition of polymers, thereby restricting the possibility for the crystallization process.48 Similarly, when curcumin is blended with these composite nanofibrous systems, no characteristic diffraction peak corresponding to Cur is observed, suggesting the amorphous nature of infused Cur.60 This kind of lowered crystallinity is indicative of better dispersion and encapsulation of Cur,63 which will also allow the improved diffusion of Cur from the nanofibrous network when required.64
Figure 3.
XRD patterns of PEO, EC, PVP, Cur, PEO-EC-Co-PEO NF, PEO-EC-Co-PVP NF, PEO-EC-Co (3%)-Cur(1%)@PEO NF, PEO-EC-Co(3%)-Cur(3%)@PEO NF, PEO-EC-Co(3%)-Cur(1%)@PVP, and PEO-EC-Co(3%)-Cur(3%)@PVP NF.
2.4. DSC Analysis
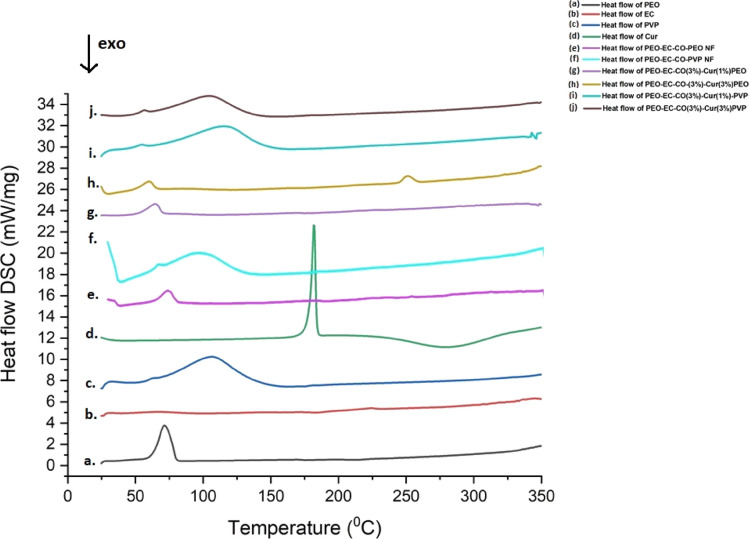
The DSC thermograms of PEO, EC, PVP, Cur, PEO-EC-Co-PEO NF, PEO-EC-Co-PVP NF, PEO-EC-Co-Cur@PEO NF, and PEO-EC-Co-Cur@PVP NF are shown in Figure 4. In the neat PEO (Figure 4a), one endothermic peak at 71.72 °C is visible, which corresponds to the melting PEO crystalline phase.65 The DSC thermogram of ethyl cellulose (Figure 4b) exhibited no peaks, thereby representing the complete amorphous nature of ethyl cellulose.66 In the case of amorphous neat PVP (Figure 4c), a broad endotherm arising from water loss can be seen at 85–150 °C.67 The sharp endothermic peak of neat curcumin appearing at 181.37 °C (Figure 4d) corresponds to the melting point of curcumin, as observed in previous studies.68 When a blend of PEO-EC-Co-PEC NF (Figure 4e) is formed, it is clearly visible that its thermogram represents more of the thermogram of PEO where the endothermic peak of melting moved to a lower value (64.59 °C). Similarly, in the case of PEO-EC-Co-PVP NF (Figure 4f) again, the water loss peak moved toward a lower temperature region (75–130 °C), which may be due the larger surface area-to-volume ratio of NFs as well as the quick arrangement of polymer molecules during electrospinning, affecting the thermodynamic and thermal properties compared to bulk polymer molecules.
Figure 4.
DSC thermograms of (a) PEO, (b) EC, (c) PVP, (d) Cur, (e) PEO-EC-Co-PEO NF, (f) PEO-EC-Co-PVP NF, (g) PEO-EC-Co(3%)-Cur (1%)@PEO NF, (h) PEO-EC-Co(3%)-Cur(3%)@PVP NF, (i) PEO-EC-Co(3%)-Cur(1%)@PVP NF, and (j) PEO-EC-Co(3%)-Cur(3%)@PVP NF.
Likewise, when considering the PEO-EC-Co-Cur@PEO NF systems (Figure 4g,h), the thermograms represent more of the PEO-EC-Co-PEO NF system, and the absence of exothermic peaks corresponding to the melting point of curcumin suggests the formation of an amorphous structure restricting the crystallization of curcumin inside the nanofiber which corroborates the XRD findings.63 In the case of PEO-EC-Co (3%)-Cur(3%)@PEO, a small endothermic peak around 250.89 °C would suggest the possible decomposition of the polymer blend due to the breaking of chemical bonds inside.69
The thermograms of PEO-EC-Co-Cur@PVP NF systems resemble more of the thermograms of PEO-EC-Co-PVP NF and PVP neat polymer where the endothermic water loss peaks of PEO-EC-Co-Cur@PVP NF systems shifted to higher temperature values (110.54 °C of PEO-EC-Co(3%)-Cur(1%)@PVP and 103.73 °C of PEO-EC-Co(3%)-Cur(3%)@PVP) compared to PEO-EC-Co-PVP NF (87.38 °C), suggesting the favorable hydrogen bonding of polymers and curcumin molecules.70
2.5. XPS Analysis
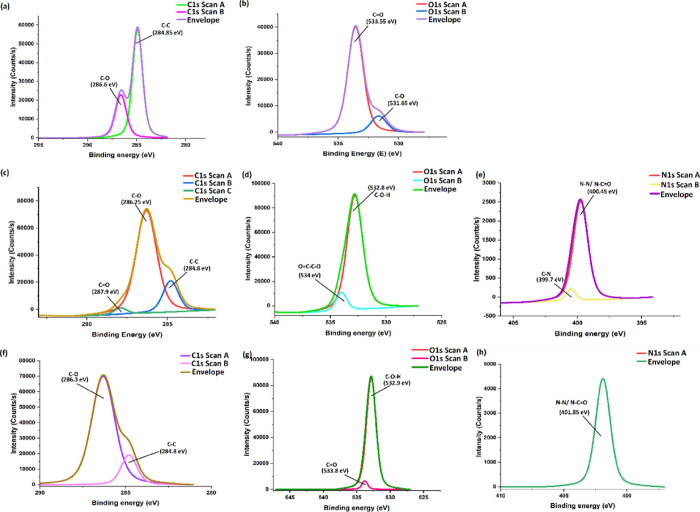
Further, XPS study was also performed to study the chemical state of the atoms on the surface of nanofibers as well as to analyze the functional groups of molecules involved in binding with curcumin molecules. The spectra of carbon (C1s) for curcumin (Figure 5a) can be deconvoluted into two peaks situated at 284.85 and 286.6 eV, corresponding to C–C and C–O, respectively.71 Similarly, the XPS spectrum of oxygen (O 1s) for curcumin (Figure 5b) was deconvoluted into two peaks and appeared at 531.65 and 533.55 eV, related to C–O and C=O vibrations, respectively.71
Figure 5.
Deconvoluted XPS spectra of (a,b) Curcumin, (c,d) PEO-EC-Co-PEO NF, and (e,f) PEO-EC-Co-Cur@PEO NF.
In the PEO-EC-Co-PEO NF system, an additional peak appears around 287.8 eV in the C1s spectrum (Figure 5c), suggesting the possible interactions built in between the −C–O–H (hydroxyl) or C=O (carbonyl) bonds that originated from the PEO, EC, and Co molecules.72−74 Moreover, a clear increment of the peak intensity corresponding to C–O bonds could be identified in the C1s spectrum of PEO-EC-CO-PEO, which indicates the contribution coming from the PEO, EC, and Co molecules in the formation of the respective nanofiber system. This was further evidenced in the corresponding O1s spectrum of PEO-EC-Co-PEO NF, which led to the increase of peak intensity corresponding to C–O bonds (Figure 5d). However, when curcumin is added during the electrospinning of the PEO-EC-Co-Cur@PEO NF, a sudden disappearance of the C–O–H/C=O bonds observed in the PEO-EC-Co-PEO neat nanofiber system can be observed (Figure 5e), which may suggest the possible hydrogen-bond interaction of C–O–H or C=O bonds originated from PEO, EC, and Co with the phenolic hydroxyl of curcumin molecules,71 which further triggers the loading of curcumin inside the nanofibrous system rather than appearing on the surface of the PEO-EC-Co-PEO nanofibers. It may further indicate the structural property rather than surface property in the PEO-EC-Co-Cur@PEO nanofibrous system.
Deconvoluted XPS spectra of PEO-EC-Co-PVP and PEO-EC-Co-Cur@PVP nanofibrous systems are indicated in Figure 6. Similar to the situation of PEO-EC-Co-PEO nanofibers being formed, here also, the appearance of three deconvoluted peaks at 284.8, 286.25, and 287.9 eV in the C 1s spectrum (Figure 6c) may suggest the presence of C–C, C–O, and C=O bonds, respectively, which originated from PEO, EC, Co, and PVP molecules.72−76 O 1s scan may indicate (Figure 6d) the presence of C–O–H bonds and O–C–C=O ester bonds at 532.8 and 534 eV, respectively.77,78 The presence of pyrrolidone-like nitrogen was evidenced by the appearance of C–N peak at 399.7 eV79 in the N 1s spectrum of the PEO-EC-Co-PVP nanofiber system. With the incorporation of curcumin, the disappearance of the C=O peak in the C1s spectrum of PEO-EC-Co-Cur@PVP (Figure 6f), which was earlier visible in the C 1s spectrum of the PEO-EC-Co-PVP NF system (Figure 6c), may further suggest the hydrogen-bonding interactions that could build up in between the C=O bonds of Co, PVP, and the phenolic −OH of curcumin, which will possibly take place at the inner interface of the PEO-EC-Co-PVP nanofibers rather than on the outer surface. Similar to the instance of curcumin being loaded into the PEO-EC-Co-PEO NF system, here also, it can be stipulated that curcumin is entrapped inside the nanofibers giving rise to a structural property rather than to a surface property. This is further evidenced by the reduction of the peak intensity and downshifting of the C=O bonds appearing in the O 1s spectrum (Figure 6g) and the disappearance of the pyrrole-like nitrogen in the N 1s spectrum of the PEO-EC-Co-Cur@PVP NF system (Figure 6h), suggesting the possible positioning and interaction of PVP molecules to load curcumin molecules into the nanofibrous system.
Figure 6.
Deconvoluted XPS spectra of (a,b) Curcumin, (c,d,e) PEO-EC-Co-PVP NF, and (f,g,h) PEO-EC-Co-Cur@PVP NF.
2.6. Assessment of Swelling, Entrapment Efficiency, and Drug Loading
Preliminary swelling could be indicative of the releasing ability of the drug molecules from the swollen nanofibrous mat because the swelling can widen up the opening of the nanofibers and would trigger the drug release. In this study, swelling of fibers was assessed in phosphate buffer (pH 5.5). As depicted in Figure S4, a rapid increase of the swelling could be seen in the first phase of the time study (2–6 h) which gets slowed down with time, finally reaching a plateau. All the nanofibrous systems exhibited a SD% over 100% even at the end of 2 h of incubation, suggesting the rapid liquid uptake ability of the fibrous systems, owing to the possible absorption via the capillary forces of the fibers in addition to the simple water absorption.80
Out of the various nanofibrous systems, PEO-EC-Co-PVP NF shows the highest SD% (683.8 ± 4.9%), followed by PEO-EC-Co(3%)-Cur(1%)@PVP(593.0 ± 4.9%) > PEO-EC-Co(3%)-Cur(3%)@PVP (569.7 ± 6.7%) > PEO-EC-Co-PEO NF (523.0 ± 8.9%) > PEO-EC-Co(3%)-Cur(1%) @PEO (483.66 ± 5.4) and then PEO-EC-Co(3%)-Cur(3%)@PEO (436.0 ± 6.2). The initial swelling of the fiber systems could be due to the favorable hydrogen bonding that takes place in between the −OH and −C=O groups present in PEO, Co, curcumin, and PVP molecules. Then, in the next step, more water molecules will get attracted around the already hydrogen-bonded water molecules which will lead to a cage-like structure with a strong hydrogen-bonding network. Later on, in the next few hours, reaching a plateau could be explained by the attraction of more free water molecules around the cage-like structure, limiting its access to the swelled inner structure of the polymeric network, thereby leading to a slight increment in swelling.81 Out of the PEO-added systems and PVP-added systems, the stronger hydrogen-bonding ability of PVP molecules must have triggered more water uptake into the fibrous structure, leading to higher SD%.82 However, the addition of curcumin would slightly affect the hydrogen-boding ability of the neat polymer molecules compared with the nanofibrous systems without curcumin due to the hydrophobic nature of curcumin and the curcumin molecules acting as a filler filling the capillary structure remaining for water absorption (the possible hydrogen-bonding pattern in the tripolymer nanofibrous structure blended with curcumin is illustrated in Figure S5). From the results, it is clear that the presence of Co and the swellability of the nanofibers co-contribute to the release of curcumin molecules.
The encapsulation efficiency (EE%) as well as the loading capacity (LD) of the systems were also analyzed. According to Table S1, it is clear that the PEO-EC-Co-Cur@PVP nanofibrous system accounts for higher EE% and LD when compared with the PEO-EC-Co-Cur@PEO system, which suggests that with the increase of the curcumin content, PVP polymer molecules trigger more favorable interactions with the curcumin molecules, thus accounting for higher EE% and LD. Moreover, in both systems, it is clear that to have better EE and LD, the increase of Co content would lead to the increase of curcumin loading into the fiber up to a certain point, whereafter further increase of Co content would competitively inhibit the loading of curcumin molecules.
2.7. Release Studies
Before conducting the release of curcumin from nanofibers infused with Co, first, the requirement of the presence of Co during the release and penetration of curcumin was identified by checking the release of curcumin from PEO-EC-Cur@PEO and PEO-EC-Cur@PVP NF which were sandwiched in between the cotton pads in the absence and presence of Co. The cumulative release percentages are indicated in Figure S6. These confirm that the release is much enhanced in the presence of Co (Co previously spread on the cotton surface), where the diffusion of curcumin from the nanofibrous matrix in both PEO-EC-PEO and PEO-EC-PVP and solubility are much triggered due to the favorable interactions made in between castor oil and curcumin.83 This highlighted the beneficiary requirement of the presence of infused Co from the nanofibers during the release and subsequent penetration of curcumin molecules.
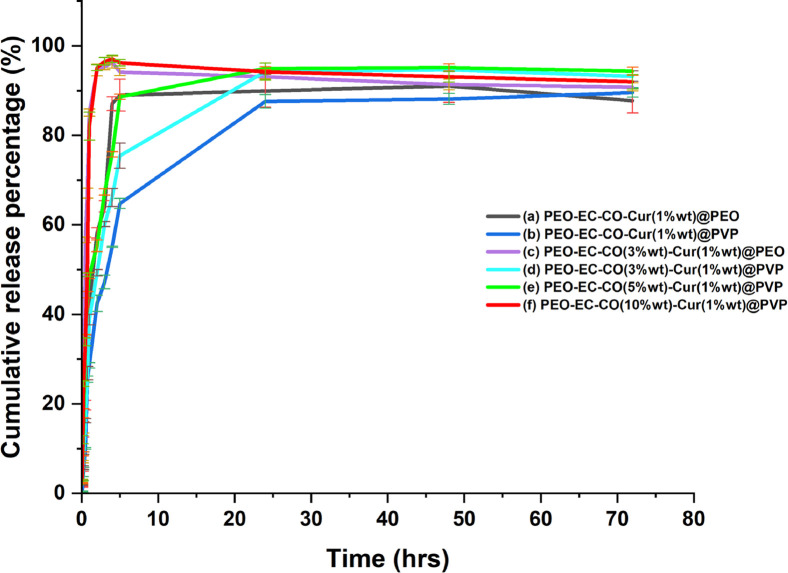
Figure 7 illustrates the cumulative release of curcumin from PEO-EC-Co-Cur@PEO and PEO-EC-Co-Cur@PVP nanofibers subjected to analysis during this study. It was noticed that when curcumin was present in higher weight % (more than 1 wt %) with a constant amount of Co, in both systems, the fibers tend to disintegrate when in contact with the release buffer (Figure S7). Therefore, the use of 1 wt % curcumin could be identified as the best curcumin percentage to attain a better releasing profile from both systems. According to Figure 7a,b, PEO-EC-Co-Cur(1 wt %)@PEO accounted for a rapid release which reached a plateau after 5 h of contact, while PEO-EC-Co-Cur(1 wt %)@PVP gave rise to a much controlled release over a period of 24 h.
Figure 7.
Cumulative release of curcumin from (a) PEO-EC-Co-Cur(1 wt %)@PEO NF, (b) PEO-EC-Co-Cur(1 wt %)@PVP NF, (c) PEO-EC-Co(3 wt %)-Cur(1 wt %)@PEO NF, (d) PEO-EC-Co(3 wt %)-Cur(1 wt %)@PVP NF, (e) PEO-EC-Co(5 wt %)-Cur(1 wt %)@PVP NF, and (f) PEO-EC-Co(10 wt %)-Cur(1 wt %)@PVP NF.
Likewise, when considering the effect of Co variation on the release of curcumin (at a constant amount of curcumin), the increase of Co in the PEO-EC-Co-Cur@PEO NF system led to a more disintegration of fibers during incubation, which could possibly result from the instability of the fibrous mat due to excessive beading. Therefore, the optimized concentration of Co for PEO-EC-Co-Cur@PEO could be identified as 3 wt %, where PEO-EC-Co(3 wt %)-Cur@PEO led to a rapid curcumin release within 4 h period (Figure 7c). In contrast, the PEO-EC-Co-Cur@PVP NF system performed well over the increment of Co content, where a controlled release of curcumin up to 24 h could be achieved (Figure 7d) when the Co content is maintained at a lower weight % (i.e., 3 wt %), while excessive increase of Co (10 wt %) led to a rapid curcumin release which shows the maximum CR% at 2 h of incubation (Figure 7d,f).
2.8. Transdermal Penetration of Released Curcumin
After careful observation of the release behavior of PEO-EC-Co-Cur@PEO and PEO-EC-Co-Cur@PVP NF systems, PEO-EC-Co(3 wt %)-Cur(1 wt %)@PEO and PEO-EC-Co(3 wt %)-Cur(1 wt %)@PVP were subjected to the transdermal penetration evaluation. As depicted in Figure S8, the development of a color in the receiver compartment clearly indicates that curcumin released from both of these nanofiber systems could penetrate effectively through the Start membrane and would reach the receiver compartment which symbolizes the blood circulation. This penetration is much faster with the curcumin released from the PEO-EC-Co(3 wt %)-Cur(1 wt %)@PEO system, which may be due to the rapid release of the total curcumin content within 4 h of incubation, whereas curcumin released from PEO-EC-Co(3 wt %)-Cur(1 wt %)@PVP showed a delayed penetration, which could be possibly due to the sustained curcumin release from the respective system.
2.9. Antioxidant Activity Via DPPH Assay
Antioxidant activity was analyzed in PEO-EC-Co(3 wt %)-Cur(1 wt %)@PEO, PEO-EC-Co(3 wt %)-Cur(3 wt %)@PEO, PEO-EC-Co(3 wt %)-Cur(1 wt %)@PVP, and PEO-EC-Co(3 wt %)-Cur(3 wt %)@PVP NFs. The color of the DPPH solution changed from purple to yellow after being reacted with nanofibrous mats, indicating the quenching of DPPH free radicals by the hydrogen donor groups in curcumin, PEO, EC, and Co, eventually leading to the formation of a stable compound. It can be deduced that the hydrogen donor groups such as the phenol groups from curcumin must have played a fundamental role in scavenging the free radicals. As given in Figure S9, the antioxidant activity of released curcumin from the electrospun mats over the immersion period of 12–48 h was evaluated, and the results indicate that the percentage inhibition ranges in between 58 and 81%. However, the antioxidant activity of as-released curcumin is decreased after a long immersion time (after 24 h). This could be possibly due to the reduced stability of curcumin with time. Compared with the control AA, a better performance over the antioxidant activity can be seen from curcumin released after 12 h from both PEO-EC-Co-Cur@PEO and PEO-EC-Co-Cur@PVP NFs. This will further suggest the residual release of curcumin from both nanofibrous samples specially can be identified with the PEO-EC-Co-Cur@PVP nanofibers. Moreover, the sustained antioxidant activity of the released curcumin further implies that the free-radical scavenging activity of curcumin remained intact even after being subjected to a high electrical potential during electrospinning. Thus, the infusion of curcumin into PEO-EC-Co-PEO or PEO-EC-Co-PVP NFs will be beneficial for curcumin to express the antioxidant properties over skin diseases, including skin cancer.
2.10. Stability Evaluation via Freeze–Thaw Cycle
The stability of the as-prepared PEO-EC-Co-Cur@PEO NF and PEO-EC-Co-Cur@PVP NFs after subjecting to a freeze–thaw cycle was evaluated by assessing the radical scavenging ability of the released curcumin. As depicted in Figure S10, it is evident that the effect of freezing, followed by thawing has a negative impact on the performance of AA, where the radical scavenging ability gradually decreased, suggesting the instability of AA over sudden temperature treatments. At the same time, only a slight reduction of the percentage of inhibition exhibited by curcumin released from nanofibrous samples suggests that the stability of curcumin is increased, and the radical scavenging ability of curcumin is intact when infused with the polymer nanofiber systems, especially when curcumin is combined with the PEO-EC-Co-PVP nanofibrous system.
3. Conclusions
Cur-loaded Co-infused PEO-EC-PEO and PEO-EC-PVP tripolymer NF systems were developed and characterized to produce transdermal skin patches with enhanced Cur release, solubility, and dermal penetration. In general, it was evident that maintaining a curcumin concentration of 1–3 wt % with 3 wt % Co in both PEO-EC-Co-Cur@PEO and PEO-EC-Co-Cur@PVP gave rise to nanofibers with lowered diameters. Moreover, the lowered crystallinity of the XRD patterns of PEO-EC-Co-Cur@PEO and PEO-EC-Co-Cur@PVP NF systems was indicative of better dispersion and encapsulation of Cur, which will potentially allow an improved diffusion of Cur from the nanofibrous network. DSC results further substantiated the XRD results due to the absence of exothermic peaks corresponding to the melting point of curcumin, suggesting the formation of an amorphous structure.
XPS data were a good source to prove the type of interaction of Cur with the PEO-EC-Co-PEO and PEO-EC-Co-PVP NF systems. In the PEO-EC-Co-Cur@PEO NF system, a sudden disappearance of the C–O–H/C=O bonds indicated the possible hydrogen-bonding interaction of C–O–H or C=O bonds originated from PEO, EC, and Co, with the phenolic hydroxyl group of curcumin molecules allowing the loading of curcumin into the nanofibrous system rather than appearing on the surface of the PEO-EC-Co-PEO nanofibers. The disappearance of the C=O peak in the C1s spectrum of the PEO-EC-Co-Cur@PVP NF further suggested the hydrogen-bonding interactions that could build up in between the C=O bonds of Co, PVP, and the phenolic −OH of curcumin, which will also provide evidence on the binding of Cur into the inner interface of the PEO-EC-CO-PVP NF than on the outer surface.
The cumulative release of Cur from PEO-EC-Co-Cur@PEO and PEO-EC-Co-Cur@PVP NFs indicated that the presence of curcumin in higher weight % (more than 1 wt %) would lead to the disintegration of NFs when in contact with the release buffer. Therefore, the use of 1 wt % Cur could be identified as the best Cur percentage to attain a better releasing profile from both systems. PEO-EC-Co-Cur(1 wt %)@PEO accounted for a rapid release, which reached a plateau after 5 h of contact, while PEO-EC-Co-Cur(1 wt %)@PVP gave rise to a much controlled release over a period of 24 h. Similarly, when considering the effect of Co on the release of Cur, it was also evident that the use of optimized concentration of 3 wt % Co is effective to attain a better Cur release in both PEO-EC-Co-Cur@PEO and PEO-EC-Co-Cur@PVP NF systems, where PEO-EC-Co(3 wt %)-Cur@PEO led to a rapid curcumin release within 4 h period, while the PEO-EC-Co-Cur@PVP NF system performed well over the increment of Co content with a controlled release of curcumin up to 24 h.
Transdermal penetration studies further confirmed that the effective dermal penetration of Cur is much enhanced with the aid of Co. Furthermore, the intact radical scavenging activity of Cur infused with PEO-EC-Co-PEO and PEO-EC-Co-PVP provides evidence that Cur could still retain its inherent properties even after being subjected to high electrical potential during electrospinning and extreme conditions during the freeze–thaw process in the presence of Co molecules. Results of this study further emphasize the beneficial role of released Cur when being applied to express the antioxidant properties over skin diseases, including skin cancer.
4. Experimental Section
4.1. Materials
PEO (Avg Mn 80,000), EC (48% ethoxy, 4 cP), PVP (Avg Mn 360,000 Da), curcumin (>98.0%, HPLC), l-ascorbic acid (L-AA), and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich (South Korea). Hydrogenated castor oil PEG 40 was purchased from biOriginds Co., Ltd. (UK). Ethanol (EtOH, ≥99.8% AnalaR NORMAPUR ACS, Reag. Ph. Eur. analytical reagent) was purchased from VWR International (USA). Phosphate saline buffer (pH 5.5) pellets were purchased from Millipore Sigma (USA). All other chemicals used were of analytical grade, and water was doubly distilled before use.
4.2. Methods
4.2.1. Preparation of Electrospinning Solution
A tripolymer-infused nanofiber system was intended to be prepared using PEO, PVP, and EC-like polymers which were subjected to monoaxial electrospinning. For the formulation of the electrospinnable polymer solution, as an empirical output of the preformulation, a polymer blend of 2 wt % PEO:EC 1:4 (w/w) was prepared in 80% ethanol solution which also contained 0.2 wt % castor oil in it. In another reaction vessel, 2 wt % PEO was mixed with curcumin to obtain 1%, 3%, and 4% w/w curcumin in PEO (Cur@PEO) solutions, respectively. Similarly, another set of polymer solutions was prepared by dissolving curcumin in 1, 3, and 4 wt % in a 5 wt % PVP in ethanol solution (Cur@PVP), similar to the Cur@PEO solutions. Respective solutions were prepared by stirring in closed vessels while subjecting to magnetic stirring for 12 h at room temperature.
In another study, the amount of Co in 2 wt % PEO:EC (1:4) was varied in the amount of 3, 5, and 10 wt %, while keeping the curcumin content at 1 wt % in both 2 wt % PEO and 5wt % PVP solutions.
Different polymer blends, curcumin, and Co used for this study are listed in Table 2.
Table 2. Different Polymer Blends Used to Electrospin Curcumin, while Varying the Concentration of Curcumin and Castor Oil.
| a constant amount of Co, while varying the curcumin content | a constant amount of curcumin, while varying the Co content | ||
|---|---|---|---|
| PEO: EC + Co (0.2 wt %) | Cur@PEO (Cur 1 wt %) | PEO: EC + Co (3 wt %) | Cur@PEO (Cur 1 wt %) |
| PEO: EC + Caster oil (0.2 wt %) | Cur@PEO (Cur 3 wt %) | PEO: EC + Caster oil (5 wt %) | Cur@PEO (Cur 1 wt %) |
| PEO: EC + Caster oil (0.2 wt %) | Cur@PEO (Cur 4 wt %) | PEO: EC + Caster oil (10 wt %) | Cur@PEO (Cur 1 wt %) |
| PEO: EC + Caster oil (0.2 wt %) | Cur@PVP (Cur 1 wt %) | PEO: EC + Caster oil (3 wt %) | Cur@PVP (Cur 1 wt %) |
| PEO: EC + Caster oil (0.2 wt %) | Cur@PVP (Cur 3 wt %) | PEO: EC + Caster oil (5 wt %) | Cur@PVP (Cur 1 wt %) |
| PEO: EC + Caster oil (0.2 wt %) | Cur@PVP (Cur 4 wt %) | PEO: EC + Caster oil (10 wt %) | Cur@PVP (Cur 1 wt %) |
4.2.2. Preparation of Curcumin Nanofibers by Electrospinning Process
The neat (PEO-EC-Co@PEO, PEO-EC-Co@PVP) and curcumin-loaded nanofibers (PEO-EC-Co-Cur@PEO and PEO-EC-Co-Cur@PVP) were made in a similar manner using a monoaxial electrospinning setup. The prepared electrospinning solutions were loaded into a 5 mL plastic syringe (Terumo) with a blunted metal needle (22 gauge). The distance between the needle tip and the aluminum foil-wrapped metal plate was adjusted to 20 cm. A high-voltage current of 16.8 kV (DC) was applied between the needle tip and the collector to initiate the charged polymer solution jet, which was rate-controlled by the syringe pump (KDS100, Cole-Parmer, USA). The feeding rate was fixed at 1.5 mL/h. The prepared nanofibers were carefully collected and stored in light-protected vacuum desiccators at room temperature, until further usage.
4.2.3. Characterizations
The surface morphology of the prepared NFs was evaluated by using a scanning electron microscope (Carl Zeiss Evo 18, Accel voltage: 10–20 kV, probe current: 1–25 pA). A portion of the nanofiber was fixed onto the stub and was subjected to gold-sputtering. Further visualization of the sample was undertaken with the use of a transmission electron microscope (JEM-2100, JEOL, Japan) operating at 200 kV. The structural changes were observed through Fourier transform infrared spectroscopy (FT-IR) using a Perkin Elmer system (Spectrum 100) in the wavelength region of 400–4000 cm–1 using the attenuated transmission (ATR) mode with 1 cm–1 resolution, and the signals were averaged from 32 scans. The PXRD patterns of the produced fibers and the starting materials were obtained using a powder X-ray diffractometer (Rigako, SmartLab SE, Japan) using Cu Kα radiation, with a step size of 2θ = 5° min–1 over the range of 2°–80°. The DSC thermograms of the nanofibers and the starting materials were measured using a differential scanning calorimeter (SETARAM DSC 131 evo) by running the samples in the range of 20–350 °C at 10 °C min–1 under a flow of nitrogen.
The samples were also subjected to XPS analysis using an EXCALABXi+ hemispherical electron energy analyzer (Thermo Fisher Scientific, USA). The incident radiation was monochromatic Al Kα X-rays (1486.6 eV) with a spot size of 900 μm. Survey scans and high-resolution scans were collected with pass energies of 150 and 20 eV and with a step size of 1.0 and 0.05 eV, respectively. All XPS measurements were performed in a constant analyzer energy (CAE) mode. Detailed spectra processing was performed by Thermo Avantage (5.982) software. The spectra were corrected for charge compensation effects by offsetting the binding energy relative to the aliphatic component of the C 1s spectrum to 284.8 eV. High-resolution spectra were resolved by fitting each peak with a combination of Gaussian/Lorentzian function after subtracting the background.
4.2.4. Degree of Swelling
The swelling of the prepared PEO-EC-Co-CR@PEO and PEO-EC-Co-CR@PVP was determined by assessing the swelling degree percentage (%). For this purpose, a specific amount of nanofibers from each system was immersed in phosphate buffer (pH 5.5). Over a period of 24 h, at specific time intervals (2, 6, 10, and 24 h), the samples were taken out, wiped to remove the surface-adhered solvent, and the weight was measured. The degree of swelling was calculated according to eq 1.
| 1 |
where Ww is the weight of the swollen nanofibers, and Wd is the dry weight of the nanofibers before immersing into the medium.
4.2.5. Drug Entrapment Efficiency and Drug Loading
The amount of entrapped drug (%) and the drug loading capacity of the drug-loaded nanofiber mat were measured by dissolving the dried mat in absolute ethanol. Then, the amount of curcumin loaded was measured by using a UV–visible spectrophotometer (Carry 300, USA) at 430 nm using eqs 2 and 3:25
| 2 |
| 3 |
4.2.6. In Vitro Drug Release Studies
4.2.6.1. Assessment of the Release of Curcumin from PEO-EC-Cur@PEO and PEO-EC-Cur@PVP in the Absence of Castor Oil When Touched with Cotton Fabrics
This specific study was conducted to evaluate the content of curcumin released from the nanofibers in the absence of castor oil when touched with a cotton fabric. For this purpose, fiber mats having a size of 5 cm × 5 cm and a weight of 0.030 g were sandwiched in between two cotton fabric pieces of the same size. Another set of fabric pieces were analyzed for the release of curcumin, which were in contact with the previously spread castor oil. In every 30 min time interval, the fabric pieces were replenished, and the curcumin content released was extracted into the ethanol medium, the absorbance of which was measured at 430 nm. This was carried out for 7 h of contact time.
4.2.6.2. Release Studies of Curcumin via Dialysis Membrane
In vitro release studies were conducted similar to our previous work.25 Briefly, a known amount of drug-loaded NF sample was placed inside a dialysis bag (MWCO of 3500) and immersed in 20.0 mL of PBS solution (pH 5.5) containing 1% (w/w) Tween 80 and gently stirred at 37 °C. At specific time intervals, 0.5 mL of the solution was withdrawn and replenished with an equal volume of fresh buffer. The amount of curcumin released into the buffer medium was determined spectrophotometrically (Carry 300, USA) at 426 nm. The release study was continued until the solution drug concentration reached an equilibrium. All experiments were carried out in triplicate. The following equation (eq 4) was used to determine the cumulative drug release percentage
| 4 |
4.2.6.3. Transdermal Diffusion of Curcumin
To assess the transdermal penetration of curcumin released through the skin, an artificial skin model (Merck Strat-M membrane) was clamped between the donor and the receptor chamber of the Franz diffusion cell with an effective permeation area of 4.9 cm2 (25 mm diameter) while having a receiver cell volume of 5.0 mL. PBS was used as both a donor and receiver solution, while the donor compartment contained 60 mg of PEO-EC-Co-Cur@PEO or PEO-EC-Co-Cur@PVP. The whole setup was incubated at 37 °C with stirring at 200 rpm. Then, 0.5 mL of sample was withdrawn at fixed time intervals (0, 2, 4, 6, 10, and 24 h), and the amount of curcumin released was measured spectrophotometrically. The solution in the receptor compartment was refilled with an equal volume of the withdrawn amount. The % permeation profile was obtained from the cumulative amount of curcumin that had permeated into the receptor compartment, and it was plotted against time.
4.2.7. Free-Radical Scavenging Ability of the PEO-EC-Co-Cur@PEO and PEO-EC-Co-Cur@PVP Fibers
The potential antioxidant activity of the curcumin-loaded nanofibers was determined based on the free-radical scavenging activity of DPPH, with some modifications made to the protocol described by Brand Williams et al.;84 200 μL aliquot of the extracted curcumin from the nanofiber pieces in ethanol was incubated with 1800 μL of 45 ppm DPPH solution prepared in ethanol. Ascorbic acid (AA) was used as a positive control. Absorbance values at 515 nm were determined after 10 min of incubation in the dark. The percent inhibition activity was calculated as follows (eq 5):
| 5 |
Curcumin was extracted by immersing the fiber mats in ethanol over a period of 12, 24, and 48 h.
4.2.8. Assessment of the Stability of the Electrospun Mats
The stability of the electrospun curcumin-loaded nanofibrous mat was evaluated using the freeze–thaw cycle test by assessing the DPPH radical scavenging activity at the end. Adapting the work suggested by Chiu et al.,51 30.0 mg of NF samples was frozen in a freezer at −20 °C for 12 h and then thawed in a normal oven at 45 °C for 8 h. After the incubation period, the remaining DPPH scavenging activity was assessed as in Section 4.2.7.
4.2.9. Statistical Evaluation
The data collected from all the experiments were coded prior to analysis and expressed in terms of mean ± standard deviation (SD), and one-way analysis of variance was used for the analysis. All statistical analyses were performed using SPSS version 19 (IBM Corporation, Armonk, NY, USA).
Acknowledgments
The authors would like to extend their sincere gratitude to the National Research Council Sri Lanka (NRC18-013) for the financial support provided. The authors are also grateful to Himala Ratnayake and the technical team at Techno Solutions Pvt., Ltd. for instrumentation support in FTIR analysis. Special appreciation is also provided to M.T.M.R. Jayaweera at the Department of Materials Science & Engineering, Faculty of Engineering, University of Moratuwa, for the instrumentation support provided in the SEM analysis.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c03495.
Detailed spectra, images, and release data (PDF)
Author Contributions
The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Fuloria S.; Mehta J.; Chandel A.; Sekar M.; Rani N. N. I. M.; Begum M. Y.; Subramaniyan V.; Chidambaram K.; Thangavelu L.; Nordin R.; Wu Y. S.; Sathasivam K. V.; Lum P. T.; Meenakshi D. U.; Kumarasamy V.; Azad A. K.; Fuloria N. K. A Comprehensive Review on the Therapeutic Potential of Curcuma Longa Linn. in Relation to Its Major Active Constituent Curcumin. Front. Pharmacol. 2022, 13, 820806 10.3389/fphar.2022.820806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X. Y.; Meng X.; Li S.; Gan R. Y.; Li Y.; Li H. B. Bioactivity, Health Benefits, and Related Molecular Mechanisms of Curcumin: Current Progress, Challenges, and Perspectives. Nutrients 2018, 10, 1553. 10.3390/nu10101553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K. M.; Dahlin J. L.; Bisson J.; Graham J.; Pauli G. F.; Walters M. A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. 10.1021/acs.jmedchem.6b00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan F. U.; Rehman M. S. U.; Khan M. S.; Ali M. A.; Javed A.; Nawaz A.; Yang C. Curcumin as an Alternative Epigenetic Modulator: Mechanism of Action and Potential Effects. Front. Genet. 2019, 10, 514. 10.3389/fgene.2019.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratrey P.; Dalvi S. V.; Mishra A. Enhancing Aqueous Solubility and Antibacterial Activity of Curcumin by Complexing with Cell-Penetrating Octaarginine. ACS Omega 2020, 5, 19004–19013. 10.1021/acsomega.0c02321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotha R. R.; Luthria D. L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. 10.3390/molecules24162930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollono L.; Falconi M.; Gaziano R.; Iacovelli F.; Dika E.; Terracciano C.; Bianchi L.; Campione E. Potential of Curcumin in Skin Disorders. Nutrients 2019, 11, 2169. 10.3390/nu11092184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim L.; Lio P. Turmeric, Curcumin, and Curcuminoids: A Dermatologic Review. Pract. Dermatol. 2020, 38–42. [Google Scholar]
- Panknin T.; Bucchireddigari B.; Howe C.; Hauer M.; Rossi A.; Funk J. A Scoping Review: Clinical Trials Assessing Turmeric Dietary Supplement Use in Cancer. FASEB J. 2021, 35, 174926. 10.1096/fasebj.2021.35.s1.05233. [DOI] [Google Scholar]
- Wei X. Q.; Zhu J. F.; Wang X. B.; Ba K. Improving the Stability of Liposomal Curcumin by Adjusting the Inner Aqueous Chamber PH of Liposomes. ACS Omega 2020, 5, 1120–1126. 10.1021/acsomega.9b03293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabanelli R.; Brogi S.; Calderone V. Improving Curcumin Bioavailability: Current Strategies and Future Perspectives. Pharmaceutics 2021, 13, 1715. 10.3390/pharmaceutics13101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothaplly S.; Alukapally S.; Nagula N.; Maddela R. Superior Bioavailability of a Novel Curcumin Formulation in Healthy Humans Under Fasting Conditions. Adv. Ther. 2022, 39, 2128–2138. 10.1007/s12325-022-02081-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urošević M.; Nikolić L.; Gajić I.; Nikolić V.; Dinić A.; Miljković V. Curcumin: Biological Activities and Modern Pharmaceutical Forms. Antibiotics 2022, 11, 135. 10.3390/antibiotics11020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn S.; Priya A.; Balasubramaniam B.; Muthuramalingam P. Biomedical Applications and Bioavailability of Curcumin — An Updated Overview. Pharmaceutics 2021, 13, 2102. 10.3390/pharmaceutics13122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang L. F.; Darwis Y.; Por L. Y.; Yam M. F. Microencapsulation Curcuminoids for Effective Delivery in Pharmaceutical Application. Pharmaceutics 2019, 11, 451. 10.3390/pharmaceutics11090451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczęsna W.; Tsirigotis-Maniecka M.; Lamch Ł.; Szyk-Warszyńska L.; Zboińska E.; Warszyński P.; Wilk K. A. Multilayered Curcumin-Loaded Hydrogel Microcarriers with Antimicrobial Function. Molecules 2022, 27, 1415. 10.3390/molecules27041415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A.; Briffa S. M.; Swingler S.; Gibson H.; Kannappan V.; Adamus G.; Kowalczuk M.; Martin C.; Radecka I. Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules 2020, 21, 1802–1811. 10.1021/acs.biomac.9b01724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjad W.; He F.; Ullah M. W.; Ikram M.; Shah S. M.; Khan R.; Khan T.; Khalid A.; Yang G.; Wahid F. Fabrication of Bacterial Cellulose-Curcumin Nanocomposite as a Novel Dressing for Partial Thickness Skin Burn. Front. Bioeng. Biotechnol. 2020, 8, 553037 10.3389/fbioe.2020.553037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algahtani M. S.; Ahmad M. Z.; Ahmad J. Nanoemulsion Loaded Polymeric Hydrogel for Topical Delivery of Curcumin in Psoriasis. J. Drug Delivery Sci. Technol. 2020, 59, 101847 10.1016/j.jddst.2020.101847. [DOI] [Google Scholar]
- Kaur N.; Garg T.; Goyal A. K.; Rath G. Formulation, Optimization and Evaluation of Curcumin-β-Cyclodextrin-Loaded Sponge for Effective Drug Delivery in Thermal Burns Chemotherapy. Drug Delivery 2016, 23, 2245–2254. 10.3109/10717544.2014.963900. [DOI] [PubMed] [Google Scholar]
- Zatorska-Płachta M.; Łazarski G.; Maziarz U.; Foryś A.; Trzebicka B.; Wnuk D.; Chołuj K.; Karewicz A.; Michalik M.; Jamroz D.; Kepczynski M. Encapsulation of Curcumin in Polystyrene-Based Nanoparticles-Drug Loading Capacity and Cytotoxicity. ACS Omega 2021, 6, 12168–12178. 10.1021/acsomega.1c00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyanka A.; Raghav N. In-Vitro Studies of Curcumin-β-Cyclodextrin Inclusion Complex as sustained release system. J. Mol. Struct. 2021, 1228, 129774 10.1016/j.molstruc.2020.129774. [DOI] [Google Scholar]
- Gupta A.; Costa A. P.; Xu X.; Lee S. L.; Cruz C. N.; Bao Q.; Burgess D. J. Formulation and Characterization of Curcumin Loaded Polymeric Micelles Produced via Continuous Processing. Int. J. Pharm. 2020, 583, 119340 10.1016/j.ijpharm.2020.119340. [DOI] [PubMed] [Google Scholar]
- Hasler-Nguyen N.; Fotopoulos G. Effect of Rubbing on the in Vitro Skin Permeation of Diclofenac-Diethylamine 1.16% Gel. BMC Res. Notes 2012, 5, 321. 10.1186/1756-0500-5-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manatunga D. C.; de Silva R. M.; de Silva K. M. N.; de Silva N.; Bhandari S.; Yap Y. K.; Costha N. P. PH Responsive Controlled Release of Anti-Cancer Hydrophobic Drugs from Sodium Alginate and Hydroxyapatite Bi-Coated Iron Oxide Nanoparticles. Eur. J. Pharm. Biopharm. 2017, 117, 29–38. 10.1016/j.ejpb.2017.03.014. [DOI] [PubMed] [Google Scholar]
- Nicolau Costa K. M.; Sato M. R.; Barbosa T. L. A.; Rodrigues M. G. F.; Medeiros A. C. D.; Damasceno B. P. G. d. L.; Oshiro-Júnior J. A. Curcumin-Loaded Micelles Dispersed in Ureasil-Polyether Materials for a Novel Sustained-Release Formulation. Pharmaceutics 2021, 13, 675. 10.3390/pharmaceutics13050675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatav V. S.; Saggu J. S.; Jat R. K.; Sharma A. K.; Singh R. P. Pharmacophore. Pharmacophore 2011, 2, 251–261. [Google Scholar]
- Joshi R.; Garud N. Development, Optimization and Characterization of Flurbiprofen Matrix Transdermal Drug Delivery System Using Box–Behnken Statistical Design. Future J. Pharm. Sci. 2021, 7, 57. 10.1186/s43094-021-00199-2. [DOI] [Google Scholar]
- Korkmaz E.; Balmert S. C.; Sumpter T. L.; Carey C. D.; Erdos G.; Falo L. D. Microarray Patches Enable the Development of Skin-Targeted Vaccines against COVID-19. Adv. Drug Delivery Rev. 2021, 171, 164–186. 10.1016/j.addr.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar R.; Ganesh M.; Senthil V.; Ramesh Y. V.; Jakki S. L.; Choi E. Y. Tetrahydro Curcumin Loaded PCL-PEG Electrospun Transdermal Nanofiber Patch: Preparation, Characterization, and in Vitro Diffusion Evaluations. J. Drug Delivery Sci. Technol. 2018, 44, 342–348. 10.1016/j.jddst.2018.01.016. [DOI] [Google Scholar]
- Sharma C. S.; Khandelwal M. A Novel Transdermal Drug-Delivery Patch for Treating Local Muscular Pain. Ther. Delivery 2018, 9, 405–407. 10.4155/tde-2018-0004. [DOI] [PubMed] [Google Scholar]
- Torres-Martinez E. J.; Cornejo Bravo J. M.; Serrano Medina A.; Pérez González G. L.; Villarreal Gómez L. J. A Summary of Electrospun Nanofibers as Drug Delivery System: Drugs Loaded and Biopolymers Used as Matrices. Curr. Drug Delivery 2018, 15, 1360–1374. 10.2174/1567201815666180723114326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzopoulou Z.; Michopoulou A.; Palamidi A.; Koliakou E.; Bikiaris D. Preparation and Evaluation of Collagen-Based Patches as Curcumin Carriers. Polymers 2020, 12, 2393. 10.3390/polym12102393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang N. W.; Kim M. H.; Sohn S. Y.; Kim K. T.; Park J. H.; Lee S. Y.; Lee J. Y.; Kim D. D. Curcumin-Loaded Lipid-Hybridized Cellulose Nanofiber Film Ameliorates Imiquimod-Induced Psoriasis-like Dermatitis in Mice. Biomaterials 2018, 182, 245–258. 10.1016/j.biomaterials.2018.08.030. [DOI] [PubMed] [Google Scholar]
- Journal A. I.; Mohammadi Z.; Zak M. S.; Majdi H.; Mostafavi E.; Barati M.; Lotfimehr H.; Ghaseminasab K.; Pazoki-Toroudi H.; Webster T. J.; Akbarzadeh A. The Effect of Chrysin–Curcumin-Loaded Nanofibres on the Wound-Healing Process in Male Rats. Artif. Cells, Nanomed., Biotechnol. 2019, 47, 1642–1652. 10.1080/21691401.2019.1594855. [DOI] [PubMed] [Google Scholar]
- Priya P.; Mohan Raj R.; Vasanthakumar V.; Raj V. Curcumin-Loaded Layer-by-Layer Folic Acid and Casein Coated Carboxymethyl Cellulose/Casein Nanogels for Treatment of Skin Cancer. Arabian J. Chem. 2020, 13, 694–708. 10.1016/j.arabjc.2017.07.010. [DOI] [Google Scholar]
- Huang Y.; Dan N.; Dan W.; Zhao W. Reinforcement of Polycaprolactone/Chitosan with Nanoclay and Controlled Release of Curcumin for Wound Dressing. ACS Omega 2019, 4, 22292–22301. 10.1021/acsomega.9b02217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirofti N.; Golandi M.; Movaffagh J.; Ahmadi F. S.; Kalalinia F. Improvement of the Wound-Healing Process by Curcumin-Loaded Chitosan/Collagen Blend Electrospun Nanofibers: In Vitro and in Vivo Studies. ACS Biomater. Sci. Eng. 2021, 7, 3886–3897. 10.1021/acsbiomaterials.1c00131. [DOI] [PubMed] [Google Scholar]
- Ahn M. Y.; Hwang J. S.; Yun E. Y.; Kim M. J.; Park K. K. Safety Evaluation of Polyethylene Glycol (PEG) Compounds for Cosmetic Use. Toxicol. Res. 2015, 31, 173–180. 10.5487/TR.2015.31.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W.Electrospinning of Ethyl Cellulose - Poly(Ethylene Oxide) and Cellulose Acetate – Poly(Ethylene Oxide) Nonwovens for the Encapsulation and Release of Thymol and Carvacrol, 2016.
- Wasilewska K.; Winnicka K. Ethylcellulose-a Pharmaceutical Excipient with Multidirectional Application in Drug Dosage Forms Development. Materials 2019, 12, 3386. 10.3390/ma12203386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmins P.; Pygall S. R.; Melia C. D.. Hydrophilic Matrix Tablets for Oral Controlled Release, 2014; Vol. 16.
- Mirzaie Z.; Barati M.; Tokmedash M. A.. Anticancer Drug Delivery Systems Based On Curcumin Nanostructures: A Review. 2020, 54 ( (4), ), 353–360, 10.1007/s11094-020-02203-0. [DOI]
- Hussain Z.; Thu H. E.; Ng S. F.; Khan S.; Katas H. Nanoencapsulation, an Efficient and Promising Approach to Maximize Wound Healing Efficacy of Curcumin: A Review of New Trends and State-of-the-Art. Colloids Surf., B 2017, 150, 223–241. 10.1016/j.colsurfb.2016.11.036. [DOI] [PubMed] [Google Scholar]
- Fereydouni N.; Darroudi M.; Movaffagh J.; Shahroodi A.; Butler A. E.; Ganjali S.; Sahebkar A. Curcumin Nanofibers for the Purpose of Wound Healing. J. Cell. Physiol. 2019, 234, 5537–5554. 10.1002/jcp.27362. [DOI] [PubMed] [Google Scholar]
- Nasery M. M.; Abadi B.; Poormoghadam D. Curcumin Delivery Mediated by Bio-Based. Molecules 2020, 25, 689. 10.3390/molecules25030689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchez A.; Acevedo F.; Cea M.; Seeger M.; Navia R. Applications of Electrospun Nanofibers with Antioxidant Properties: A Review. Nanomaterials 2020, 10, 175. 10.3390/nano10010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahma A.; Munir M. M.; Khairurrijal; Prasetyo A.; Suendo V.; Rachmawati H. Intermolecular Interactions and the Release Pattern of Electrospun Curcumin-Polyvinyl(Pyrrolidone) Fiber. Biol. Pharm. Bull. 2016, 39, 163–173. 10.1248/bpb.b15-00391. [DOI] [PubMed] [Google Scholar]
- He Y.; Liu H.; Bian W.; Liu Y.; Liu X.; Ma S.; Zheng X.; Du Z.; Zhang K.; Ouyang D. Molecular Interactions for the Curcumin-Polymer Complex with Enhanced Anti-Inflammatory Effects. Pharmaceutics 2019, 11, 442. 10.3390/pharmaceutics11090442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianluca V.; Elena L.; Vittoria V.; Giuliana G. Coaxial Electrospun Membranes of Poly(ε-Caprolactone)/Poly (Lactic Acid) with Reverse Core-Shell Structures Loaded with Curcumin as Tunable Drug Delivery Systems. Polym. Adv. Technol. 2021, 32, 4005–4013. 10.1002/pat.5404. [DOI] [Google Scholar]
- Chiu C.; Nootem J.; Santiwat T.; Srisuwannaket C. Enhanced Stability and Bioactivity of Curcuma Comosa Roxb. Extract in Electrospun Gelatin Nanofiber. Fibers 2019, 7, 76. 10.3390/fib7090076. [DOI] [Google Scholar]
- Villa Nova M.; Gonçalves M. D. C. P.; Nogueira A. C.; Herculano L. D. S.; Medina A. N.; Bazotte R. B.; Bruschi M. L. Formulation and Characterization of Ethylcellulose Microparticles Containing.l-Alanyl- l-Glutamine Peptide. Drug Dev. Ind. Pharm. 2014, 40, 1308–1317. 10.3109/03639045.2013.817417. [DOI] [PubMed] [Google Scholar]
- Panhwar T.; Mahesar S. A.; Kandhro A. A.; Sheerazi S. T. H.; Kori A. H.; Laghari Z. H.; Memon J.-R. Physicochemical Composition and FTIR Characterization of Castor Seed Oil. Ukr. Food J. 2019, 8, 778–787. 10.24263/2304-974x-2019-8-4-9. [DOI] [Google Scholar]
- Abdelrazek E. M.; Abdelghany A. M.; Badr S. I.; Morsi M. A. Evaluation of Optical Parameters and Structural Variations of UV Irradiated (PEO/PVP)/Au Polymer Nanocomposites. Res. J. Pharm., Biol. Chem. Sci. 2016, 7, 1877–1890. [Google Scholar]
- Zidan H. M.; Abdelrazek E. M.; Abdelghany A. M.; Tarabiah A. E. Characterization and Some Physical Studies of PVA/PVP Filled with MWCNTs. J. Mater. Res. Technol. 2019, 8, 904–913. 10.1016/j.jmrt.2018.04.023. [DOI] [Google Scholar]
- Baganizi D. R.; Nyairo E.; Duncan S. A.; Singh S. R.; Dennis V. A. Interleukin-10 Conjugation to Carboxylated PVP-Coated Silver Nanoparticles for Improved Stability and Therapeutic Efficacy. Nanomaterials 2017, 7, 165. 10.3390/nano7070165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail E. H.; Sabry D. Y.; Mahdy H.; Khalil M. M. H. Synthesis and Characterization of Some Ternary Metal Complexes of Curcumin with 1,10-Phenanthroline and Their Anticancer Applications. J. Sci. Res. 2014, 6, 509–519. 10.3329/jsr.v6i3.18750. [DOI] [Google Scholar]
- Rezaei A.; Nasirpour A. Encapsulation of Curcumin Using Electrospun Almond Gum Nanofibers: Fabrication and Characterization. Int. J. Food Prop. 2018, 21, 1608–1618. 10.1080/10942912.2018.1503300. [DOI] [Google Scholar]
- Machmudah S.; Winardi S.; Kanda H.; Goto M.. Formation of Fine Particles from Curcumin/PVP by the Supercritical Antisolvent Process with a Coaxial Nozzle. 2020, 12, 6705, 10.1021/acsomega.9b04495. [DOI] [PMC free article] [PubMed]
- Wang C.; Ma C.; Wu Z.; Liang H.; Yan P.; Song J.; Ma N.; Zhao Q. Enhanced Bioavailability and Anticancer Effect of Curcumin-Loaded Electrospun Nanofiber: In Vitro and In Vivo Study. Nanoscale Res. Lett. 2015, 10, 439. 10.1186/s11671-015-1146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anilkumar K. M.; Jinisha B.; Manoj M.; Jayalekshmi S. Poly(Ethylene Oxide) (PEO) – Poly(Vinyl Pyrrolidone) (PVP) Blend Polymer Based Solid Electrolyte Membranes for Developing Solid State Magnesium Ion Cells. Eur. Polym. J. 2017, 89, 249–262. 10.1016/j.eurpolymj.2017.02.004. [DOI] [Google Scholar]
- Cellulose T. E.; Cellulose M.; Trivedi M. K.; Branton A.; Trivedi D.; Nayak G. Characterization of Physicochemical and Thermal Properties of Biofield Treated Ethyl Cellulose and Methyl Cellulose. Int. J. Biomed. Mater. Res. 2015, 3, 83–91. 10.11648/j.ijbmr.20150306.12. [DOI] [Google Scholar]
- Ravikumar R.; Ganesh M.; Ubaidulla U.; Young Choi E.; Tae Jang H. Preparation, Characterization, and in Vitro Diffusion Study of Nonwoven Electrospun Nanofiber of Curcumin-Loaded Cellulose Acetate Phthalate Polymer. Saudi Pharm. J. 2017, 25, 921–926. 10.1016/j.jsps.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal R.; Sarwar Alam M.; Gupta B. Preparation of Curcumin Loaded Poly(Vinyl Alcohol)-Poly(Ethylene Oxide)-Carboxymethyl Cellulose Membranes for Wound Care Application. J. Biomater. Tissue Eng. 2013, 3, 273–283. 10.1166/jbt.2013.1092. [DOI] [Google Scholar]
- Poly E.; Oxide E.; Rosero M. I. D.; Meneses N. M. J.; Uribe R. Thermal Properties of Composite Polymer Electrolytes Poly(Ethylene Oxide)/Sodium Trifluoroacetate/Aluminum Oxide (PEO)10CF3COONa + x Wt.% Al2O3. Materials 2019, 12, 1464. 10.3390/ma12091464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindasamy G.; Kannan Krishnamoorthy M. R. Selection Of Excipients For Nanoparticles Formulations Of Nateglinide Through Drug-Excipients Compatibility Study. Int. J. Pharm. Pharm. Sci. 2015, 5, 371–377. [Google Scholar]
- Umayangana Godakanda V.; Li H.; Alquezar L.; Zhao L.; Zhu L.-M.; Rohini de Silva K. M.; Nalin de Silva G. R. W. Tunable Drug Release from Blend Poly(Vinyl Pyrrolidone)-Ethyl Cellulose Nanofibers. Int. J. Pharm. 2019, 562, 172–179. 10.1016/j.ijpharm.2019.03.035. [DOI] [PubMed] [Google Scholar]
- Hu L.; Shi Y.; Li J. H.; Gao N.; Ji J.; Niu F.; Chen Q.; Yang X.; Wang S. Enhancement of Oral Bioavailability of Curcumin by a Novel Solid Dispersion System. AAPS PharmSciTech 2015, 16, 1327–1334. 10.1208/s12249-014-0254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor N. A.; Einbond L. S.; Redenti S.; Moira Sauane A. J. A Self-Degradable Curcumin Polymer with Anti-Cancer Activity. J. Appl. Polym. Sci. 2018, 135, 46867. 10.1002/app.46867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L.; Zhang K.; Ishida H.; Froimowicz P. Study of the Effects of Intramolecular and Intermolecular Hydrogen-Bonding Systems on the Polymerization of Amide-Containing Benzoxazines. Macromol. Chem. Phys. 2017, 218, 1600562 10.1002/macp.201600562. [DOI] [Google Scholar]
- Khandelwal P.; Alam A.; Choksi A.; Chattopadhyay S.; Poddar P. Retention of Anticancer Activity of Curcumin after Conjugation with Fluorescent Gold Quantum Clusters: An in Vitro and in Vivo Xenograft Study. ACS Omega 2018, 3, 4776–4785. 10.1021/acsomega.8b00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. M. D.; Hewitt J. A.; Meenan B. J. X-ray Photoelectron Spectroscopy and Infra-red Studies of X-ray-induced Beam Damage of Cellulose, Ethyl Cellulose and Ethyl-hydroxyethyl Cellulose. Surf. Interface Anal. 1992, 18, 199–209. 10.1002/sia.740180305. [DOI] [Google Scholar]
- Thomas H. R.; O’Malley J. J. Surface Studies on Multicomponent Polymer Systems by X-Ray Photoelectron Spectroscopy. Polystyrene/Poly (Ethylene Oxide) Diblock Copolymers. Macromolecules 1979, 12, 323–329. 10.1021/ma60068a033. [DOI] [Google Scholar]
- Zeng Q.; Dong G.; Martin J. M. Green Superlubricity of Nitinol 60 Alloy against Steel in Presence of Castor Oil. Sci. Rep. 2016, 6, 29992. 10.1038/srep29992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Jiang P.; Jiang M.; Wang T. W.; Guo C. F.; Xie S. S.; Wang Z. L. The Shape Evolution of Gold Seeds and Gold@silver Core-Shell Nanostructures. Nanotechnology 2009, 20, 305602 10.1088/0957-4484/20/30/305602. [DOI] [PubMed] [Google Scholar]
- Morales M. E.; Ruiz M. A.; Oliva I.; Oliva M.; Gallardo V. Chemical Characterization with XPS of the Surface of Polymer Microparticles Loaded with Morphine. Int. J. Pharm. 2007, 333, 162–166. 10.1016/j.ijpharm.2006.09.051. [DOI] [PubMed] [Google Scholar]
- Jia R.; Chen J.; Zhao J.; Zheng J.; Song C.; Li L.; Zhu Z. Synthesis of Highly Nitrogen-Doped Hollow Carbon Nanoparticles and Their Excellent Electrocatalytic Properties in Dye-Sensitized Solar Cells. J. Mater. Chem. 2010, 20, 10829–10834. 10.1039/c0jm01799j. [DOI] [Google Scholar]
- López G. P.; Castner D. G.; Ratner B. D. XPS O 1s Binding Energies for Polymers Containing Hydroxyl, Ether, Ketone and Ester Groups. Surf. Interface Anal. 1991, 17, 267–272. 10.1002/sia.740170508. [DOI] [Google Scholar]
- Osadchii D. Y.; Olivos-Suarez A. I.; Bavykina A. V.; Gascon J. Revisiting Nitrogen Species in Covalent Triazine Frameworks. Langmuir 2017, 33, 14278–14285. 10.1021/acs.langmuir.7b02929. [DOI] [PubMed] [Google Scholar]
- Tsuge M.; Takahashi K.; Kurimoto R.; Fulati A.; Uto K.; Kikuchi A.; Ebara M. Fabrication of Water Absorbing Nanofiber Meshes toward an E Ffi Cient Removal of Excess Water from Kidney Failure Patients. Fibers 2019, 7, 39. 10.3390/fib7050039. [DOI] [Google Scholar]
- Gunathilake T. M. S. U.; Ching Y. C.; Chuah C. H. Enhancement of Curcumin Bioavailability Using Nanocellulose Reinforced Chitosan Hydrogel. Polymers 2017, 9, 64. 10.3390/polym9020064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne E.; Worku Z. A.; Healy A. M. Physicochemical Properties of Poly-Vinyl Polymers and Their Influence on Ketoprofen Amorphous Solid Dispersion Performance: A Polymer Selection Case Study. Pharmaceutics 2020, 12, 433. 10.3390/pharmaceutics12050433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjana D.; Anitha Nair K.; Somashekara N.; Venkata M.; Sripathy R.; Yelucheri R.; Parmar H.; Upadhyay R.; Rama Verma S.; Ramchand C. N. Development of Curcumin Based Ophthalmic Formulation. Am. J. Infect. Dis. 2012, 8, 41–49. 10.3844/ajidsp.2012.41.49. [DOI] [Google Scholar]
- Brand-Williams W.; Cuvelier M. E.; Berset C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT–Food Sci. Technol. 1995, 28, 25–30. 10.3906/sag-1411-35. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.