Abstract
Plants of the genus Strobilanthes have notable use in folklore medicines as well as being used for pharmacological purposes. The present work explored the biological predispositions of Strobilanthes glutinosus and attempted to accomplish a comprehensive chemical profile through GC-MS of different fractions concerning polarity (chloroform and n-butanol) and LC-ESI-MS of methanolic extract by both positive and negative ionization modes. The biological characteristics such as antioxidant potential were assessed by applying six different methods. The potential for clinically relevant enzyme (α-amylase, α-glucosidase, and tyrosinase) inhibition was examined. The DPPH, ABTS, CUPRAC, and FRAP results revealed that the methanol fraction presented efficient results. The phosphomolybdenum assay revealed that the n-hexane fraction showed the most efficient results, while maximum metal chelation potential was observed for the chloroform fraction. The GC-MS profiling of n-butanol and chloroform fractions revealed the existence of several (110) important compounds presenting different classes (fatty acids, phenols, alkanes, monoterpenes, diterpenes, sesquiterpenoids, and sterols), while LC-ESI-MS tentatively identified the presence of 44 clinically important secondary metabolites. The n-hexane fraction exhibited the highest potential against α-amylase (497.98 mm ACAE/g extract) and α-glucosidase (605.85 mm ACAE/g extract). Significant inhibitory activity against tyrosinase enzyme was displayed by fraction. Six of the prevailing compounds from the GC-MS study (lupeol, beta-amyrin, stigmasterol, gamma sitosterol, 9,12-octadecadienoic acid, and n-hexadecanoic acid) were modelled against α-glucosidase and α-amylase enzymes along with a comparison of binding affinity to standard acarbose, while three compounds identified through LC-ESI-MS were docked to the mushroom tyrosinase enzyme and presented with significant biding affinities. Thus, it is assumed that S. glutinosus demonstrated effective antioxidant and enzyme inhibition prospects with effective bioactive molecules, potentially opening the door to a new application in the field of medicine.
Keywords: Strobilanthes glutinosus, antioxidant, enzyme inhibition, tyrosinase inhibition, GC-MS, LC-ESI-MS, docking
1. Introduction
Medicinal plants have shown substantial medicinal and therapeutic benefits, due to which they are becoming important worldwide. Plants have become an object of ample importance in research as well as alternative medicinal therapy [1]. The rapidly increasing population and poverty in the developing world hamper this population from availing of high-priced pharmaceutical products. Medicinal plants are their main source for health care delivery. Around 70–80% of the developing world depends on conventional remedies obtained from medicinal plants [2]. Several novel compounds have been isolated from plants and have demonstrated unique and interesting biological activities [3]. The current research focus is to extract pharmacologically active compounds from natural provenance that can be helpful, particularly in the area of diseases that presently lack an effective medicinal therapy.
There is a major shift of attention from modern medicine to parallel herbal systems, leading to a revival of alternative medicines [4]. According to an estimate, drugs derived from natural sources account for 20–25% of all drugs which are mentioned in the Pharmacopeia. Several medicinal plants are being employed for disease management without any modification [5]. The study of disease progression and induction has shown that oxidative stress is a major causative agent of various diseases. Chronic accumulation of reactive oxygen species causes cellular oxidative stress which ultimately leads to disease progression. Antioxidants of plant origin exhibit great potential; therefore, therapeutic focus has shifted towards the herbal medicine [6]. Phytochemicals possess great antioxidant activity that contributes to the therapeutic efficacy of plants [7].
Strobilanthes is a genus belonging to the family Acanthaceae, comprising around 350 species [8]. In this genus, the majority of plants present with anti-inflammatory and wound healing properties. These also show potential antimicrobial, anti-diabetic, and anti-cancer activities. Their extracts have been effective in spider poisoning, influenza epidemic, cerebrospinal meningitis, viral pneumonia, mumps, and acute respiratory syndrome [9]. Even though pharmacological research has described a broad variety of biological activities and chemical properties of the Strobilanthes genus (See Supplementary Materials), several species of the genus remain unexplored. S. glutinosus is a species that has not been studied scientifically in terms of biological and chemical properties. Only one study regarding the antimicrobial and antioxidant activity of this plant has been conducted in the Department of Botany, Mirpur University of Science and Technology (MUST), Mirpur-10250 (AJK), Pakistan [10].
Therefore, the current work was designed to conduct the chemical analysis to evaluate the bioactive content and GC-MS analysis of different extracts in order to analyze the phytochemical composition. The biological potential was studied by performing antioxidant assays of hydro-methanol extract and n-butanol, chloroform, and n-hexane fractions of the whole plant of S. glutinosus. Additionally, the present work proposed to determine the inhibitory effect of key enzymes (alpha-glucosidase and alpha-amylase) involved in diabetes mellitus along with molecular docking studies to explore any probable interaction between observed secondary metabolites and reported enzyme inhibition results. Molecular docking became an imperative tool for searching for inhibitor interactions at the receptor’s active site. Docking studies, to calculate binding free energy, also reveal the most appropriate confirmation that aids in the development of novel inhibitors against targeted enzymes. In terms of the literature evaluation, this study may be considered the preliminary analysis of the phytochemical composition, antioxidant properties, enzyme inhibition, and molecular docking studies of selected compounds from GC-MS of S. glutinosus.
2. Results
2.1. Phytochemical Composition
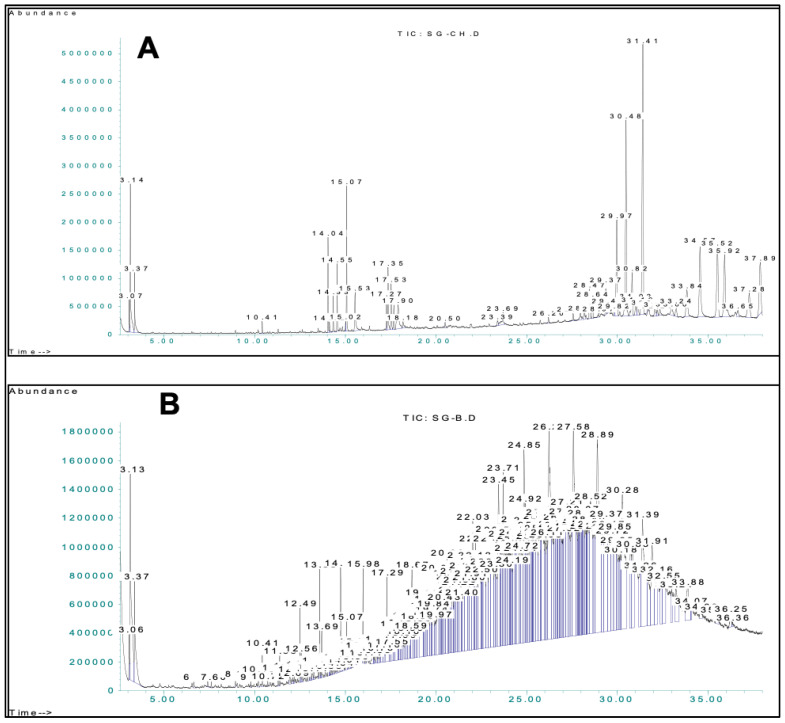
In this recent work, two different extracts of S. glutinosus were assessed for their bioactive contents via GC-MS, as presented in Table 1 and Table 2 and Figure 1, which enabled the tentative identification of 110 compounds. This GC-MS phytochemical investigation of different extracts of S. glutinosus can be considered the first comprehensive study.
Table 1.
GC-MS analysis of chloroform fraction of S. glutinosus.
| Sr. | RT | % Area | Name of Compound | Mol. Weight | Mol. Formula | Chem. Class |
|---|---|---|---|---|---|---|
| 1 | 3.07 | 0.69 | Ethylbenzene | 106 | C8H10 | Alkylbenzene |
| 2 | 3.14 | 4.14 | Benzene, 1,3-dimethyl- | 160 | C8H4Cl6 | Alkylbenzene |
| 3 | 3.37 | 1.75 | p-Xylene | 106.16 | C8H10 | Hydrocarbon |
| 4 | 10.41 | 0.27 | Phenol, 2,5-bis(1,1-dimethylether | 206.32 | C14H22O | Phenol |
| 5 | 14.12 | 0.29 | 2-Pentadecanone, 6,10,14-trimet, … | 268.5 | C18H36O | Sesquiterpenoids |
| 6 | 14.33 | 0.90 | 9-Octadecene | 252.5 | C18H36 | Hydrocarbon |
| 7 | 15.02 | 0.27 | 2-Cyclopenten-1-one, 2-pentyl- | 152.3 | C10H16O | Cyclic ketones |
| 8 | 15.07 | 3.57 | Hexadecanoic acid, methyl ester | 270.5 | C17H34O2 | Fatty acid |
| 9 | 17.27 | 0.94 | 9,12-Octadecadienoic acid, meth, … | 294.5 | C19H34O2 | Fatty acid |
| 10 | 17.90 | 2.40 | (R)-(-)-14-Methyl-8-hexadecyn-1-ol | 252.4 | C17H32O | Hydrocarbon |
| 11 | 18.18 | 0.41 | 2-Piperidinone, N-[4-bromo-n-bu, … | 234.1 | C9H16BrNO | Delta-lactams |
| 12 | 27.56 | 0.34 | Pyridine-3-carboxamide, oxime, … | 137.4 | C6H7 N3O | Oxime |
| 13 | 27.97 | 0.44 | 2-Ethylacridine | 207.2 | C15H13N | Acridine |
| 14 | 28.18 | 0.47 | Cyclotrisiloxane, hexamethyl- | 222.4 | C6H18O3Si3 | Organosilicon |
| 15 | 28.47 | 1.33 | Eicosane | 282.5 | C20H42 | Alkane |
| 16 | 28.64 | 0.74 | Cholesta-6,22,24-triene, 4,4-di, … | 394.7 | C29H46 | Sterol |
| 17 | 29.37 | 1.18 | 1,3,5-Trisilacyclohexane, 1,1-d, … | 339.0 | C3H6Cl6Si3 | Hetrocyclic |
| 18 | 29.97 | 5.53 | Cholest-5-en-3-ol (3.beta.)-, c, … | 386.7 | C27H46O | Cholesterol |
| 19 | 30.82 | 2.42 | Ergosta-4,6,22-trien-3.beta.-ol | 396.6 | C28H44O | Sterol |
| 20 | 31.02 | 0.85 | Phenylacetic acid, 2-(1-adamant, … | 298.4 | C2oH26O2 | Ethyl ester |
| 21 | 31.12 | 0.74 | Benz[b]-1,4-oxazepine-4(5H)-thi, … | 207.2 | C11H13NOS | Alkyl benzene |
| 22 | 32.35 | 0.43 | 2,4-Cyclohexadien-1-one, 3,5-bi, … | 184.1 | C12H8O2 | Cyclohexadien |
| 23 | 33.00 | 0.77 | 1H-Indole, 1-methyl-2-phenyl- | 207.2 | C15H13N | Phenyl indole |
| 24 | 33.24 | 0.76 | 1-Bromoeicosane | 361.4 | C20H41Br | Alkane |
| 25 | 33.84 | 2.29 | Campesterol | 400.7 | C28H48O | Sterol |
| 26 | 35.92 | 5.74 | Stigmasterol, 22,23-dihydro- | 412.7 | C29H48O | Sterol |
| 27 | 36.65 | 0.46 | beta.-Amyrin | 426.7 | C30H50O | Triterpenoid |
| 28 | 37.28 | 2.52 | Lup-20(29)-en-3-one | 424.7 | C30H48O | Triterpenoid |
| 29 | 37.89 | 4.58 | Lupeol | 426.7 | C30H50O | Triterpenoid |
Table 2.
GC-MS analysis of n-butanol fraction of S. glutinosus.
| Sr. | RT | % Area | Name of Compound | Mol. Weight | Mol. Formula | Chem. Class |
|---|---|---|---|---|---|---|
| 1 | 3.06 | 0.13 | Ethylbenzene | 106.1 | C8H10 | Ar. hydrocarbon |
| 2 | 3.13 | 1.31 | p-Xylene | 106.1 | C8H10 | Ar. hydrocarbon |
| 3 | 3.37 | 0.64 | o-Xylene | 106.1 | C8H10 | Ar. hydrocarbon |
| 4 | 6.63 | 0.03 | m-Mentha-4,8-diene, (1S,3S)-(+)- | 136.2 | C10H16 | Ar. hydrocarbon |
| 5 | 7.40 | 0.01 | 1H-Inden-1-one, 2,3-dihydro-3,4, … | 174.2 | C12H14O | Indanones |
| 6 | 7.60 | 0.01 | Decane, 3,8-dimethyl- | 170.3 | C12H26 | Ali. hydrocarbon |
| 7 | 8.94 | 0.02 | 1-Tetradecene | 196.3 | C14H28 | Ali. hydrocarbon |
| 8 | 9.77 | 0.02 | Nonadecane | 268.5 | C19H40 | Ali. hydrocarbon |
| 9 | 10.19 | 0.05 | Pentacosane | 352.7 | C20H52 | Ali. hydrocarbon |
| 10 | 10.42 | 0.09 | Phenol, 2,5-bis(1,1-dimethyleth, … | 206.3 | C14H22O | Phenol |
| 11 | 10.71 | 0.01 | Octacosane | 394.8 | C28H58 | Ali. hydrocarbon |
| 12 | 11.30 | 0.03 | 1-Hexadecene | 224.4 | C16H32 | Ali. hydrocarbon |
| 13 | 11.38 | 0.06 | Hexadecane | 226.4 | C16H34 | Ali. hydrocarbon |
| 14 | 11.93 | 0.05 | 2-Undecene, 5-methyl- | 168.32 | C12H24 | Ali. hydrocarbon |
| 15 | 12.08 | 0.02 | Hexadecane, 2-methyl- | 240.5 | C17H36 | Ali. hydrocarbon |
| 16 | 12.18 | 0.03 | Pentadecane | 212.4 | C15H32 | Ali. hydrocarbon |
| 17 | 12.49 | 0.16 | Heptadecane | 240.5 | C17H36 | Ali. hydrocarbon |
| 18 | 12.56 | 0.08 | Pentadecane, 2,6,10,14-tetramet, … | 268.5 | C19H40 | Ali. hydrocarbon |
| 19 | 12.63 | 0.04 | Hentriacontane | 436.8 | C31H64 | Ali. hydrocarbon |
| 20 | 12.97 | 0.05 | Tetratetracontane | 619.2 | C44H90 | Ali. hydrocarbon |
| 21 | 13.18 | 0.04 | Heptadecane, 2-methyl- | 215.4 | C18H38 | Ali. hydrocarbon |
| 22 | 13.27 | 0.03 | Heptadecane, 3-methyl- | 254.9 | C18H38 | Ali. hydrocarbon |
| 23 | 13.51 | 0.03 | 1-Octadecene | 252.6 | C18H36 | Ali. hydrocarbon |
| 24 | 13.70 | 0.14 | Hexadecane, 2,6,10,14- phytane) | 282.5 | C20H42 | Diterpene |
| 25 | 14.12 | 0.02 | 7-Oxabicyclo [4.1.0]heptane, 1,5, … | 194.2 | C12H18O2 | Ali. hydrocarbon |
| 26 | 14.16 | 0.03 | Tetradecane, 5-methyl- | 212.4 | C15H32 | Ali. hydrocarbon |
| 27 | 14.24 | 0.02 | Pentadecane | 212.4 | C15H32 | Ali. hydrocarbon |
| 28 | 14.54 | 0.05 | Tetrapentacontane, 1,54-dibromo- | 917.2 | C54H108Br2 | Ali. hydrocarbon |
| 29 | 14.66 | 0.05 | Nonadecane, 9-methyl- | 282.5 | C20H42 | Ali. hydrocarbon |
| 30 | 14.84 | 0.02 | Cyclotetradecane, 1,7,11-trimet, … | 280.5 | C20H40 | Diterpene |
| 31 | 15.07 | 0.13 | Pentadecanoic acid, 14-methyl-, … | 256.4 | C16H32O2 | Fatty acid |
| 32 | 15.12 | 0.02 | 7,9-Di-tert-butyl-1-oxaspiro(4, … | 276.4 | C17H24O3 | Flavanoids |
| 33 | 15.36 | 0.02 | Octadecane, 1-chloro- | 288.9 | C18H37Cl | Alkyl chloride |
| 34 | 15.58 | 0.02 | Cyclopentadecane | 210.4 | C15H30 | Alkane |
| 35 | 15.90 | 0.03 | 1-Nonadecene | 266.5 | C19H38 | Ali. hydrocarbon |
| 36 | 16.48 | 0.30 | Heneicosane | 296.6 | C21H44 | Ali. hydrocarbon |
| 37 | 16.73 | 0.04 | Octadecane | 254.5 | C18H38 | Ali. hydrocarbon |
| 38 | 16.91 | 0.08 | Nonadecane | 268.5 | C19H40 | Ali. hydrocarbon |
| 39 | 16.98 | 0.11 | Cycloeicosane | 280.5 | C20H40 | Alkane |
| 40 | 17.46 | 0.14 | 1-Docosene | 308.6 | C22H44 | Ali. hydrocarbon |
| 41 | 17.55 | 0.12 | 2-Eicosanol, (.+/−.)- | 298.5 | C20H42O | Phenol |
| 42 | 17.69 | 0.16 | tert-Hexadecanethiol | 258.2 | C16H34S | Thiol |
| 43 | 18.39 | 0.17 | Tridecane, 6-cyclohexyl- | 266.5 | C19H38 | Ar. hydrocarbon |
| 44 | 18.50 | 0.21 | Hexadecanoic acid, butyl ester | 312.5 | C20H40O2 | Fatty acid ester |
| 45 | 19.19 | 0.72 | Nonahexacontanoic acid | 999.8 | C69H138O2 | Fatty acid |
| 46 | 19.31 | 0.19 | Nonadecane, 1-chloro- | 303 | C19H39Cl | Alkane |
| 47 | 19.97 | 0.24 | Docosane | 310.6 | C22H46 | Alkane |
| 48 | 20.22 | 0.33 | Tricosane | 324.6 | C23H48 | Alkane |
| 49 | 20.28 | 0.23 | Cyclotetradecane, 1,7,11-trimet, … | 280.5 | C20H40 | Alkane |
| 50 | 20.35 | 0.32 | Nonadecane, 1-chloro- | 302.9 | C19H39Cl | Alkane |
| 51 | 20.43 | 0.40 | 1-Chloroeicosane | 317.0 | C20H41Cl | Alkyl halide |
| 52 | 20.61 | 0.81 | Docosane | 310.6 | C22H46 | Alkane |
| 53 | 20.69 | 0.24 | Octadecane | 254.5 | C18H38 | Alkane |
| 54 | 21.41 | 0.21 | Hexadecane, 1-iodo- | 352.34 | C16H33I | Alkyl halide |
| 55 | 21.64 | 1.01 | 1-Chloroeicosane | 317.0 | C20H41Cl | Alkyl halide |
| 56 | 21.80 | 0.38 | 1-Tricosene | 322.6 | C23H46 | Alkene |
| 57 | 21.91 | 0.74 | 1-Nonadecene | 266.5 | C19H38 | Alkene |
| 58 | 22.03 | 1.06 | Hexadecane, 1-iodo- | 352.34 | C16H33I | Alkyl Halide |
| 59 | 22.66 | 1.01 | 1-Hexacosene | 364.7 | C 26H52 | Alkene |
| 60 | 22.95 | 0.99 | Pentacosane | 352.7 | C25H52 | Alkane |
| 61 | 23.07 | 1.24 | Hexacosane | 366.71 | C26H54 | Alkane |
| 62 | 23.45 | 1.46 | Nonadecane, 9-methyl- | 282.5 | C20H42 | Alkane |
| 63 | 23.54 | 0.45 | Hexadecane, 2-methyl- | 240.5 | C17H36 | Alkane |
| 64 | 23.71 | 1.05 | Di-n-octyl phthalate | 390.6 | C24H38O4 | Benzoic acid esters |
| 65 | 24.05 | 0.32 | Nonahexacontanoic acid | 999.8 | C69H138O2 | Fatty acid |
| 66 | 24.12 | 0.46 | Ethanol, 2-(octadecyloxy)- | 314.5 | C20H42O2 | Phenol |
| 67 | 24.19 | 0.26 | 1-Chloroeicosane | 317.0 | C20H41Cl | Alkyl halide |
| 68 | 24.26 | 1.01 | Octadecane | 254.4 | C18H38 | Alkane |
| 69 | 24.73 | 0.77 | 1-Decanol, 2-hexyl- | 242.44 | C16H34O | Alchohol |
| 70 | 24.85 | 1.41 | Nonadecane, 9-methyl- | 282.5 | C20H42 | Alkane |
| 71 | 24.91 | 0.79 | Octadecane, 1-iodo- | 380.4 | C18H37I | Alkyl halide |
| 72 | 26.12 | 0.74 | Tricosane | 324.6 | C23H48 | Alkane |
| 73 | 26.24 | 2.61 | Heptacosane, 1-chloro- | 415.2 | C27H55Cl | Alkyl halide |
| 74 | 27.21 | 1.36 | Heptacosane | 380.7 | C27H56 | Alkane |
| 75 | 27.58 | 2.56 | Octacosane | 394.7 | C28H58 | Alkane |
| 76 | 28.89 | 3.64 | Eicosane | 282.5 | C20H42 | Ali. hydrocarbon |
| 77 | 31.40 | 2.01 | Heneicosane, 3-methyl- | 310.6 | C22H46 | Alkane |
| 78 | 33.24 | 0.45 | 1-Bromoeicosane | 361.4 | C20H41Br | Alkyl Halide |
| 79 | 34.60 | 0.09 | Z-14-Nonacosane | 406.8 | C29H58 | Alkanes |
| 80 | 35.49 | 0.08 | Methoxyacetic acid, heptadecyl, … | 314.5 | C19H38O3 | Ester |
| 81 | 36.25 | 0.11 | Tetratriacontane, 17-hexadecyl- | 703.3 | C50H102 | Alkanes |
Figure 1.
GC-MS chromatograms of chloroform (A) and n-butanol (B) fractions.
To gain a more in-depth insight into the phytochemical composition through the LC-ESI-MS method, we looked into the phytochemicals present in the plant S. glutinosus in greater detail. Due to its many benefits, including low solvent consumption, high precision, and accuracy, the hybrid coupled technique is frequently employed for the investigation of phytochemicals derived from plants [11].
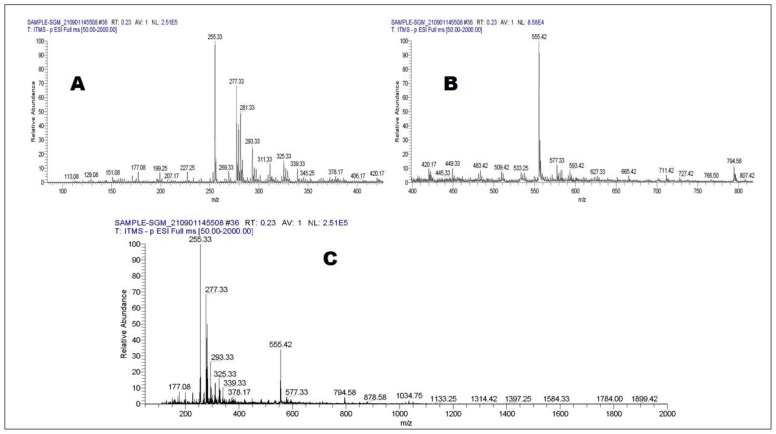
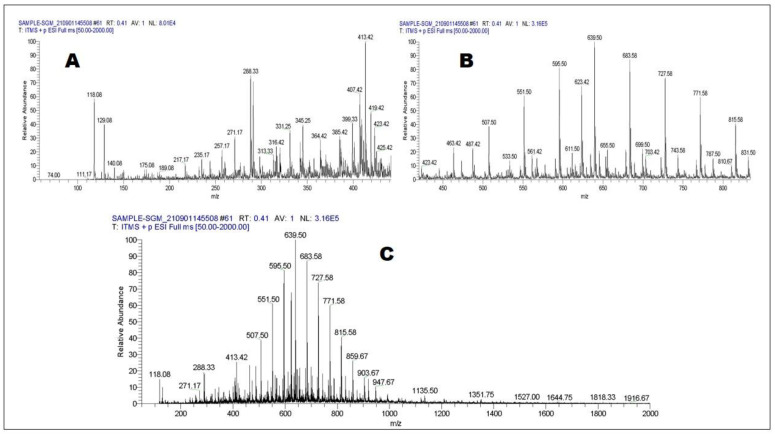
Positive and negative ionizing modes of LC-ESI-MS-MS were used to monitor the profile of secondary metabolites, resulting in the identification of 44 compounds (Table 3 and Table 4). Phenols, phenolic acids, phenolic glycosides, flavonoids, flavonoid glucoside, fatty acids, triterpenoids, lignans, and coumarin are just some of the chemical classes represented by the compounds identified. Figure 2 and Figure 3 represents total ion chromatograms of both negative and positive ionization modes.
Table 3.
LC-ESI-MS-MS screening of Strobilanthes glutinosus in negative mode.
| Sr. | RT (min) | % Area | Tentative Identification | Mol. Formula | Mol. Mass | Adduct | Chemical Class |
|---|---|---|---|---|---|---|---|
| 1 | 10.95 | 0.49 | Aesculetin | C9H6O4 | 177 | [−H] | Coumarin |
| 2 | 11.70 | 0.22 | Echinospine | C10H9NO | 159 | [−H] | Other |
| 3 | 11.91 | 1.57 | Syringic acid | C9H10O5 | 199 | [−H] | Phenol |
| 4 | 12.25 | 0.36 | Daidzein | C15H10O4 | 253 | [−H] | Flavonoid |
| 5 | 12.46 | 5.84 | Hispidulin | C16H12O6 | 255 | [−H] | Flavonoid |
| 6 | 12.70 | 0.47 | Emmotin A | C16H22O4 | 277 | [−H] | Terpenoid |
| 7 | 2.21 | 0.55 | p-coumaryl malic acid | C13 H12 O7 | 279 | [−H] | Phenol |
| 8 | 13.06 | 2.74 | Oleic acid | C18H34O2 | 281 | [−H] | Fatty acid |
| 9 | 2.37 | 0.33 | Catechin | C15H14O6 | 289.5 | [−H] | Phenol |
| 10 | 13.32 | 1.15 | Gingerol | C17C26O4 | 293.50 | [−H] | Phenol |
| 11 | 2.63 | 0.25 | 8-Prenylnaringenin | C20H20O5 | 295.00 | [HCOO] | Flavonoid |
| 12 | 13.40 | 0.68 | Caffeoyl tartaric acid | C13H12 O9 | 311.00 | [−H] | Phenolic acid |
| 13 | 13.61 | 0.94 | p-coumaric acid hexoside | C15H18O8 | 325.00 | [−H] | Phenolic acid |
| 14 | 14.09 | 1.28 | Sesamolinol | C20H20O7 | 371.00 | [−H] | Lignan |
| 15 | 14.29 | 1.16 | Oleuropein aglycone | C19H20O8 | 377 | [−H] | Phenol |
| 16 | 14.49 | 0.93 | Campesterol | C28H48O | 400 | [−H] | Terpenoid |
| 17 | 14.71 | 1.58 | Beta-amyrin | C30H50O | 425 | [−H] | Terpenoid |
| 18 | 15.01 | 0.33 | (−)-Epicatechin 3-O-gallate | C22H18O10 | 441.50 | [−H] | Flavonoid |
| 19 | 15.99 | 1.88 | Myricetin 3-O-arabinoside | C20H18O12 | 449.50 | [−H] | Flavonoid |
| 20 | 16.16 | 1.46 | 3-Hydroxyphloretin 2′-O-glucoside | C21H24O11 | 451.15 | [−H] | Glucoside |
| 21 | 16.34 | 4.32 | Lariciresinol-sesquilignan | C30H36O10 | 555.00 | [−H] | Lignan |
| 22 | 16.76 | 1.76 | Pratensein 7-O-β-d-glucoside 6″O-malonate | C25H23O13 | 549.50 | [−H] | Flavonoid |
| 23 | 17.05 | 0.49 | Luteolin 7- rutinoside | C27H30O15 | 593 | [−H] | Flavonoid |
Table 4.
LC-ESI-MS-MS screening of Strobilanthes glutinosus in positive mode.
| Sr. | RT (min) | % Area | Tentative Identification | Mol. Formula | Mol. Mass | Adduct | Chemical Class |
|---|---|---|---|---|---|---|---|
| 1 | 1.50 | 1.36 | Betaine | C5H11NO2 | 118.00 | [+H] | Amino acid |
| 2 | 2.28 | 0.94 | Gentesic acid | C7H6O4 | 156.00 | [+H] | Phenolic acid |
| 3 | 2.71 | 1.44 | Azelaic acid | C9H16O4 | 189.00 | [+H] | Dicarboxylic acid |
| 4 | 3.06 | 2.57 | Angustifoline | C14H22N2O | 235.00 | [+H] | Alkaloid |
| 5 | 0.57 | 0.66 | Apigenin | C15H10O5 | 271.00 | [+H] | Flavonoid |
| 6 | 0.95 | 0.35 | Linoleinic acid | C18H30O2 | 279.17 | [+H] | Fatty acid |
| 7 | 1.24 | 0.62 | Linoleic acid | C18H32O2 | 281.50 | [+H] | Fatty acid |
| 8 | 3.43 | 0.68 | Eriodictyol | C15H12O6 | 289.00 | [+H] | Flavonoid |
| 9 | 3.60 | 0.44 | Catechin | C15H14O6 | 291.00 | [+H] | Phenol |
| 10 | 3.98 | 0.27 | Gallic acid hexoside | C13H16O10 | 331.50 | [+H] | Phenolic glycoside |
| 11 | 4.28 | 0.38 | 7dehydro cholesterin | C27H44O | 385 | [+H] | Terpenoid |
| 12 | 5.90 | 1.68 | α-tocopherol | C29H50O2 | 429.30 | [+H] | Terpenoid |
| 13 | 6.21 | 1.74 | 5-OH liquiritin | C21H22O10 | 435.30 | [+H] | Flavonoid |
| 14 | 6.99 | 2.89 | Ligstroside | C25H32O12 | 523.30 | [+H] | Phenolic glycoside |
| 15 | 6.42 | 2.33 | Scutellarin | C21H18O12 | 463.30 | [+H] | Flavonoid |
| 16 | 7.33 | 1.46 | Agnuside | C22H26O11 | 467.50 | [+H] | Other |
| 17 | 7.57 | 5.18 | di-O-acetyldarutoside | C30H48O10 | 567.50 | [+H] | Phenol |
| 18 | 7.87 | 4.78 | Rutin | C27H30O16 | 611.20 | [+H] | Flavonoid |
| 19 | 8.32 | 4.56 | Quercetin-6,4′-dimethoxy-3-fructo-rhamnoside | C21H20O11 | 655.50 | [+H] | Flavonoid |
| 20 | 8.84 | 7.23 | Quercetin rhamnoside-feruloyl-hexoside | C31H28O15 | 743.55 | [+H] | Flavonoid |
| 21 | 9.97 | 5.99 | Quercetin 3-O-rhamnosyl-glucoside 7-O-rhamnoside | C27H30O16 | 875.50 | [+H] | Flavonoid |
Figure 2.
LC-ESI-MS-MS full scan of Strobilanthes glutinosus (negative mode) 50–400 (A), 50–800 (B), and 50–2000 (C).
Figure 3.
LC-ESI-MS-MS full scan of Strobilanthes glutinosus (positive mode) 50–400 (A), 50–800 (B), and 50–2000 (C).
2.2. Antioxidant Assays
In the present study, six different methods (DPPH, ABTS, FRAP, CUPRAC, phosphomolybdenum, and metal chelating assays) were used to determine the antioxidant potential of S. glutinosus, and Table 5 furnishes the results of the study.
Table 5.
Antioxidant results by different methods of S. glutinosus whole plant extract/fractions.
| Extract/Fractions | Radical Scavenging Assays | Reducing Power Assays | Total Antioxidant Capacity | Ferrous Ion Chelation | ||
|---|---|---|---|---|---|---|
| DPPH (mg TE/g Extract) |
ABTS (mg TE/g Extract) |
CUPRAC (mg TE/g Extract) |
FRAP (mg TE/g Extract) |
Phosphomolybdenum (mg TE/g Extract) |
Metal Chelation (mg EDTAE/g Extract) |
|
| Methanol | 56.217 ± 0.66 a | 63.469 ± 0.045 a | 245.116 ± 4.240 a | 87.126 ± 0.083 a | 96.015 ± 0.476 b | 17.038 ± 0.0769 b |
| n-butanol | 47.920 ± 0.166 c | 47.669 ± 0.078 c | 162.629 ± 6.372 b | 75.097 ± 0.054 b | 6.544 ± 0.748 d | 7.692 ± 0.0769 d |
| Chloroform | 50.130 ± 0.108 b | 53.574 ± 5.183 b | 84.693 ± 2.780 c | 65.007 ± 0.361 c | 60.698 ± 0.079 c | 12.384 ± 8.423 c |
| n-hexane | 9.804 ± 1.234 d | 12.761 ± 0.045 d | 59.878 ± 4.865 d | 34.971 ± 1.820 d | 119.587 ± 0.555 a | 25.346 ± 0.192 a |
All of the procedures were carried out thrice. The mean ± standard deviation were used to represent the results. Trolox and EDTAE were utilized as standard. Significantly different results were exhibited when compared to standard (p < 0.05). Superscripts (a–d) represents statistical difference.
2.3. In Vitro Enzyme Inhibition Activity
The studied plant extracts were tested against different enzymes, including α-amylase, α-glucosidase, and tyrosinase. The standard used for α-amylase and α-glucosidase was acarbose, and the results were presented in mmol ACAE/g extract. Kojic acid was used as the standard for tyrosinase enzyme, with results being presented in mg KAE/g extract. The inhibitory potential of plant extract/fractions against all three enzymes is displayed in Table 6. Maximum percentage inhibition against α-amylase and α-glucosidase was displayed by chloroform fraction (501.407 ± 2.982 and 605.854 ± 6.252 mmol ACAE/g extract), respectively. While methanolic extract exhibited the highest potential against tyrosinase enzyme (9.86 ± 1.41 mg KAE/g extract).
Table 6.
Enzyme inhibition results of S. glutinosus whole plant extract/fractions.
| Extract/Fractions | α-Amylase (mmol ACAE/g Extract) |
α-Glucosidase (mmol ACAE/g Extract) |
Tyrosinase (mg KAE/g Extract) |
|---|---|---|---|
| Methanol | 166.758 ± 1.72 b | 294.195 ± 3.036 b | 9.86 ± 1.4 a |
| n-butanol | 107.007 ± 1.104 c | 118.64 ± 1.224 c | 8.52 ± 1.82 b |
| Chloroform | 501.407 ± 2.982 a | 605.854 ± 6.252 a | 6.91 ± 1.35 c |
| n-hexane | 85.859 ± 0.510 d | 93.572 ± 0.965 d | NA |
All of the procedures were carried out thrice. The mean ± standard deviation were used to represent the results. ACAE: acarbose equivalent, KAE: kojic acid equivalent. Significantly different results were exhibited when compared to standard (p < 0.05). NA (No Activity). Superscripts (a–d) represents statistical difference.
2.4. In Silico Analysis
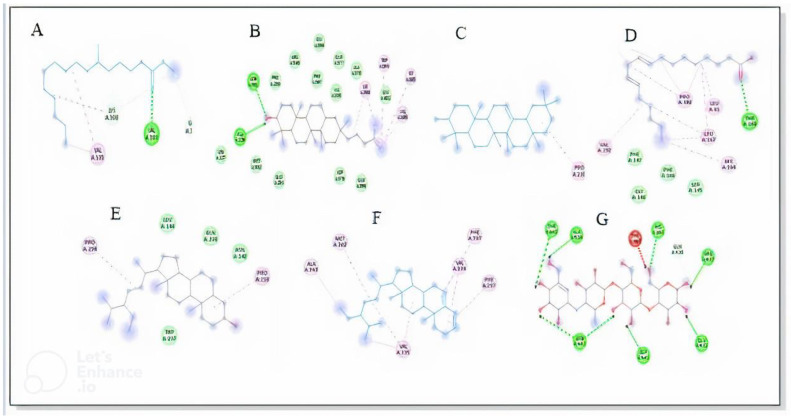
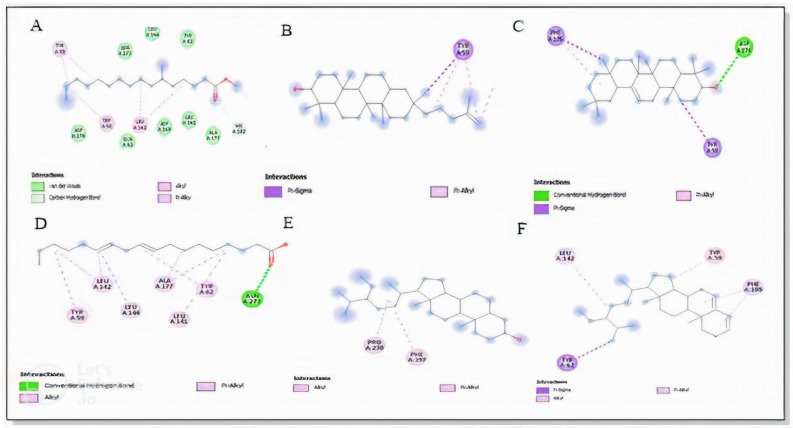
A total of 29 compounds identified in GC-MS analysis of the chloroform fraction were docked, and six of them were selected based on their binding affinities along with the standard compound acarbose against the receptor α-glucosidase and α-amylase enzymes. PubChem, the drug database, was used for downloading the 3D structure of ligand molecules. Beta-amyrin, sitosterol, stigmasterol, and lupeol were identified to be the most suitable ligands, with significant binding affinities. Our results indicated that beta amyrin had the highest binding affinity with the α glucosidase enzyme, with a docking score of –8.4 kcal/mol, followed by stigmasterol (−7.5 kcal/mol), sitosterol (−7.5 kcal/mol), lupeol (−6.9 kcal/mol), 9,12-octadecadienoic acid (−4.1 kcal/mol), and n-hexadecanoic acid (−3.5 kcal/mol), presented in Table 7 and Figure 4 and Figure 5. Beta amyrin, sitosterol, and stigmasterol presented the highest binding affinity versus α glucosidase enzyme, with a binding energy of −8.4 and −7.5 kcal/mol, respectively, ranking higher in comparison to standard drug acarbose (−6.6 kcal/mol).
Table 7.
Binding affinities and interactions of ligands against (anti-diabetic) enzymes.
| Enzyme | Ligands | Binding Energy (kcal/mol) | Electrostatic/Hydrophobic Interaction |
|---|---|---|---|
| α-glucosidase | Acarbose | −6.6 | Hydrogen bond (Thr448, Asn443, Ala514, Asp441, Glu432, Arg437, His348) C-H bond (Gln438) |
| Lupeol | −6.9 | Alkyl interaction (Lys398, Trp394, Val380, Trp354) Hydrogen bond (Ala229, Asn301) Van-dar walls (Pro230, Arg340, Phe357, Ala378, Gly402, Glu377, Val335, Leu227, Met302, Glu396, Asp379, Glu231) |
|
| Sitosterol | −7.5 | Alkyl interaction (Leu45, Ala444) C-H bond (Leu433) Van-dar walls (Ala434, Arg450, Met407, Thr445) |
|
| 9,12-octadecadienoic acid | −4.1 | Alkyl interaction (Leu446) Van-dar walls (Asp411, Thr410, Leu373, Leu45, Asp440, Ser44, Gln438, Pro408, Glu432, Leu431) |
|
| β-Amyrin | −8.4 | Alkyl interaction (Pro230) | |
| Hexadacanoic acid | −3.9 | Hydrogen bond (Aal380) C-H bond (Lyc398, Gly998) Alkyl interaction (Val335) |
|
| Stigmasterol | −7.5 | Alkyl interaction (Val335, Ala343, Met302, Val334, Phe397, Phe297) | |
| α-amylase | Lupeol | −7.6 | Pi-sigma (Tyr59) Pi-alkyl (Trp60) |
| Sitosterol | −5.1 | Pi-alkyl (Pro230, Phe397) | |
| 9,12-octadecadienoic acid | −4.9 | Hydrogen bond (Asn273) Alkyl interaction (Tyr59, Leu142, Met302, Ala177, Leu141) |
|
| β-Amyrin | −8.4 | Hydrogen bond (Asp274) Pi-sigma (Phe105) Pi-alkyl (Tyr59) |
|
| Hexadacanoic acid | −4.6 | Van-dar walls (Asp274, Tyr62, Ala177, Asp176, Gln63, Asp269, Leu144, Asn273) Pi-alkyl (Tyr59, Trp58, Leu142) C-H bond (His102, Gln208) |
|
| Stigmasterol | −9.1 | Pi-sigma (Tyr59) Pi-alkyl (Tyr59, Leu142, Phe105) |
Figure 4.
Enzyme α-glucosidase and ligands interaction. Hexadecanoic acid (A), Lupeol (B), β-Amyrin (C), Octadecadienoic acid (D), Sitosterol (E), Stigmasterol (F), Acarbose (standard) (G).
Figure 5.
Enzyme α-amylase and ligands interaction. Hexadecanoic acid (A), Lupeol (B), β-Amyrin (C), Octadecadienoic acid (D), Sitosterol (E), Stigmasterol (F).
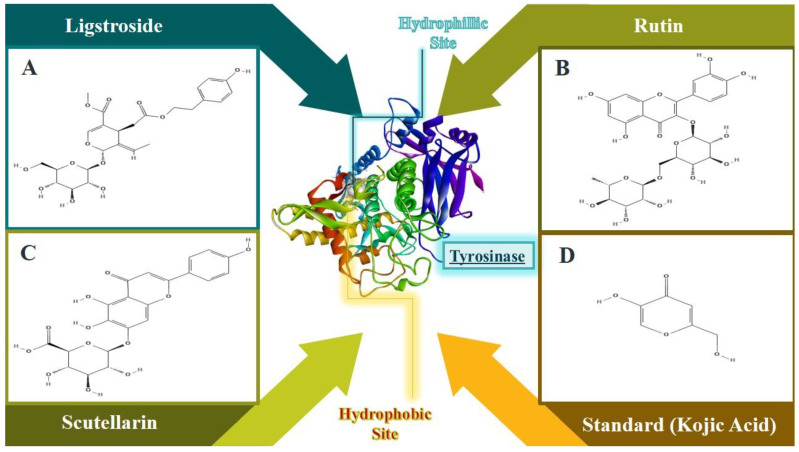
Molecular docking on three key compounds (Lingstroside, Rutin, and Scutellarin) identified from the methanolic extract using LC-ESI-MS (Figure 6) was performed against mushroom tyrosinase enzyme. As a reference drug for such conditions, kojic acid was also included in the assay. The binding affinities and amino acid interactions are presented in Table 8 and Figure 7.
Figure 6.
Molecular docking of selected ligands with tyrosinase enzyme. (A) Ligstroside, (B) Rutin, (C) Scutellarin, and (D) Kojic acid.
Table 8.
Binding affinities and interactions of the selected ligands from S. glutinosus extract by LC-ESI-MS against tyrosinase enzyme.
| Enzyme | Ligand | Binding Affinity (Kcal/mol) | Amino Acids Interactions |
|---|---|---|---|
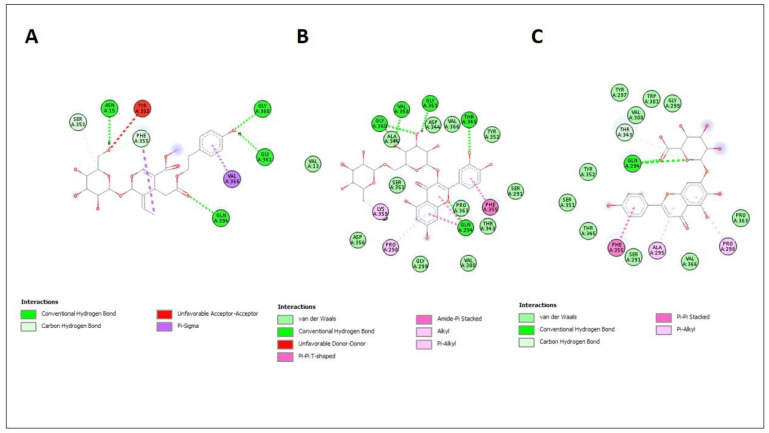
| Tyrosinase | Ligstroside | −8.0 | Unfavorable Accaptor: (TYRA352) Pi Sigma: (VALA366) Conventional Hydrogen Bond: (ASNA15, GLNA294, GLYA360, GLYA361) Carbon Hydrogen: (SERA351, PHEA355) |
| Rutin | −8.9 | Amide-Pi Stacked: (PHEA355) Pi-Alkyl: (PROA298, LYSA359) Conventional Hydrogen Bond: (GLNA294, THRA345, VALA358, GLYA360, GLYA361) Van der Waals: (VALA13, GLYA299, VALA300, THRA343, ASPA344, ALAA346, SERA351, TYRA352, PROA363, VALA366) |
|
| Scutellarin | −8.6 | Pi-Pi Stacked: (PHEA355) Pi-Alkyl: (ALAA295, PROA298) Conventional Hydrogen Bond: (GLNA294) Carbon Hydrogen: (THRA343) Van der Waals: (SERA291, TYRA297, GLYA299, VALA300, TRPA301, THRA345, SERA351, TYRA352, PROA363, VALA366) |
|
| Kojic acid (Standard) |
−5.3 | Pi-Pi Stacked: (PHEA355) |
Figure 7.
Enzyme tyrosinase and ligands interaction. Lingstroside (A), Rutin (B), Scutellarin (C).
3. Discussion
Bioactive chemicals such as those found in plants are crucial to human health because they stimulate cell division and repair, two processes essential to being healthy as a whole [12]. There is evidence in the literature that indicates S. glutinosus methanol extract has significant flavonoid and phenolic levels [10]. Overall, 29 and 81 major compounds were identified from chloroform and n-butanol fractions, respectively. From 110 compounds, n-hexadecanoic acid, 9,12-Octadecadienoic acid (2,2)-, methyl ester, linoelaidic acid, 11,13-dimethy1-12-tetradecane-1-olacetate, heptadecanal, alpha-tocospiro A, alpha-tocospiro B, dl- stigmasterol, gamma–sitosterol, lup-20(29)-en-3-one, lupeol, and linoleic acid were identified as major bioactive compounds from the whole-plant extract. Lupeol (sterol) is one of the major compounds detected in high concentrations in the GC-MS study. Lupeol is found naturally in edible fruits and vegetables and is reported to have antioxidant, anti-diabetic, hepatoprotective, anti-inflammatory, anti-protozoal, anti-microbial, anti-proliferative, and cholesterol-lowering effects [13]. Another compound having a reported anticancer activity and antioxidant effect is 9, 12-Octadecenoic acid methyl ester (Z, Z), fatty acid methyl ester [14].
The pharmacological benefits of flavonoids and phenols are well-documented. Many studies have shown that phenols and flavonoids are effective antioxidants, anti-inflammatory agents, and enzyme inhibitors, with significant clinical uses [15,16,17,18]. In the present study (LC-ESI-MS), a total of 14 flavonoids were identified, 6 in negative mode and 8 in positive mode of ionization. Deprotonated molecules [M-H]− were observed in Daidzein (Rt = 12.25 min), Hispidulin (Rt = 12.46), 8-Prenylnaringenin (Rt = 2.63), (−), Epicatechin 3-O-gallate (Rt = 15.01), Myricetin 3-O-arabinoside (Rt = 15.99), and Luteolin 7- rutinoside (Rt = 17.05), while Apigenin (Rt = 0.57 min), Eriodictyol (Rt = 3.43), 5-OH liquiritin (Rt = 6.21), scutellarin (Rt = 6.42), and Rutin (Rt = 7.87) displayed ions’ positive mode of ionisation. Similarly, 10 phenols and their derivatives were tentatively identified in both the negative and positive mode of ionisation analysis. Syringic acid (Rt = 11.91), p-coumaryl malic acid (Rt = 2.21), catechin (Rt = 2.37), and gingerol (Rt = 13.32) are the phenols found, and all of them showed deprotonated molecules [M-H]−. In the present chromatographic analysis of the S. glutinosus methanol fraction, three phenolic acids, caffeoyl tartaric acid (Rt = 13.40), p-coumaric acid hexoside (Rt = 13.61), and gentesic acid (Rt = 2.28), were tentatively identified. Additionally, catechin was detected in the positive ionisation mode, where it showed a peak at m/z 291.00. A single phenolic glycoside, gallic acid hexoside (Rt = 3.98), was tentatively identified in the positive mode of ionization by displaying the protonated molecule [M+H]+ at 331.50. In the process, two lignans, sesamolinol (Rt = 14.09) and lariciresinol-sesquilignan (Rt = 16.34), were isolated in the negative mode of the analysis. Emmotin A, a terpenoid (Rt = 12.70), was detected in the negative mode, as were campesterol (Rt = 14.49) and beta amyrin (Rt = 14.71), while tocopherol (Rt = 5.90) was detected in the positive mode.
The oxidative stress caused by reactive oxygen species has been linked to the pathogenesis of a wide variety of degenerative illnesses [19]. Endogenous antioxidants with exogenous antioxidants mostly derived from plants prevent the oxidative stress [20] Plants are the main source of antioxidant compounds. Consequently, for oxidant-induced diseases, research has been focused on plants [19]. Antioxidant effects cannot be established by using a single method since plant extracts contain a large number of chemicals that comprise antioxidant activity with a varied mechanism of action [21]. For these reasons, different methods were applied to analyze the antioxidant activity of plant extracts.
The hydro-methanol extract of S. glutinosus expressed the highest value in the DPPH assay (56.21 mg TE/g extract) and ABTS assay (63.46 mg TE/g extract) TE/g extract. The n-butanol and chloroform fractions shared almost similar results in both assays. whereas the lowest effect was measured towards the n-hexane fraction (DPPH: 9.80 mg TE/g extract) and (ABTS: 12.76 mg TE/g extract).
The extract’s antioxidant activity is also significant because of its reducing power. By reducing the ferric tripridyltriazine complex to the ferrous complex at low pH, the FRAP assay measures the antioxidant’s capacity to contribute electrons to reduce ferric ions. The hydro-methanol extract presented higher reducing power (87.12 mg TE/g extract), while the n-hexane fraction exhibited the lowest reducing potential (34.97 mg TE/g extract). The n-butanol and chloroform fractions shared almost similar results, i.e., 75.09 and 65.00 mg TE/g extract, respectively. In the results for the CUPRAC assay, the hydro-methanol extract exhibited higher values (245.11 mg TE/g extract) following the order from hydro-methanol > n-butanol > chloroform > n-hexane. There was a link between free radical scavenging assays and reducing power assays, suggesting that the bioactive content results verified the increased amount of phenolic and flavonoid components in methanol and butanol extracts [22].
Furthermore, a phosphomolybdenum assay was used to evaluate the total antioxidant capacity, and the findings are furnished in Table 2. The n-hexane fraction of S. glutinosus exhibited the highest total antioxidant capacity (119.58 mg TE/g extract), while hydro-methanol extract and chloroform fraction exhibited considerable total antioxidant capacity potential, with values of 96.01 and 60.69 mg TE/g extract, respectively. The n-butanol fraction was the least active fraction for this assay (6.54 mg Trolox equivalent/g). The presence of non-phenolic compounds with chelating properties among phytoconstituents is consistent with other findings reported by [23]. The GC-MS study of S. glutinosus presented the compounds thymol, lupeol, alpha-tocopherol, and squalene, having antioxidant activity reported by [24,25,26], which justifies the results.
In developing nations, where Type 2 diabetes mellitus accounts for 90% of all cases, the prevalence of diabetes mellitus is anticipated to more than double, from 171 million in 2000 to 300 million by 2025 [27]. Popular antidiabetic drugs such as acarbose, voglibose, and miglitol all work by inhibiting alpha-amylase and alpha-glucosidase enzymes, resulting in lower blood glucose levels. However, these drugs have undesirable side effects, including toxicity to the liver and gastrointestinal issues, when used long-term [28]. Consequently, there is a demand for novel alpha-glucosidase and alpha-amylase inhibitors derived from natural origins, particularly from herbs and plants that produce no unpleasant or undesirable side effects in diabetic patients.
The results of alpha-amylase, alpha-glucosidase, and tyrosinase inhibition assays of different fractions of S. glutinosus are presented in Table 6. Among the tested extracts, chloroform fraction was most efficient against α-amylase (501.407 ± 2.98 mmol ACAE/g extract) and α-glucosidase enzyme (605.854 ± 6.252 mmol ACAE/g extract). Likewise, the hydro-methanol extract was also noticeably active against both (amylase and glucosidase) enzymes, with the values of 166.758 ± 1.721 and 294.195 ± 3.036 mmol ACAE/g extract, respectively. The evaluated anti-diabetic potential of S. glutinosus complies with the earlier reports as described in [29], an in vivo assay of S. cuspidata to evaluate the anti-diabetic potential and the isolated compounds. Lupein, (3-Hydroxy-4-methoxy phenyl) cinnamic acid and stigmasterol exhibited confirmed antidiabetic potential by inhibiting α amylase enzyme [29].
Melanin biosynthesis, also called melanogenesis, is a physiological process that is catalysed in humans by the enzyme tyrosinase [30]. Tyrosinase inhibitors may be useful for treating dermatological disorders associated with melanin hyperpigmentation [31], as they work by reducing the activity of tyrosinase, an enzyme that is responsible for the production of melanin. Tyrosinase inhibition can also be useful for the food industry. However, preventing tyrosinase activity is ideal for preserving the freshness of fruits and vegetables for a longer period of time. The methanolic extract showed prominent activity against the tyrosinase enzyme with a value of 9.86 ± 1.41 mg KAE/g extract. The tyrosinase inhibition results of S. glutinosus extracts were ordered as follows: methanol > n-butanol > chloroform > n-hexane. Studies have shown that various phenolics and flavonoids (as revealed via LC-ESI-MS of S. glutinosus methanol extract) have anti-tyrosinase potenial.
To accurately anticipate the ligand–target binding energy and to offer an understanding of the molecular-based mechanism of biological processes that ligands produced, computational techniques have been successfully employed in the pharmaceutical and nutraceutical industries. More information on how physiologically active chemicals can bind to certain enzymes can be gleaned through molecular docking studies [32]. The ligand molecules were lupeol, beta amyrin, stigmasterol, gamma sitosterol, 9,12-octadecadienoic acid, and n-hexadecanoic acid. The energy and stability of the conformer were then minimized before docking to obtain the lowest energy and a more stable conformer. The binding of certain proteins with ligands promotes the efficiency of biological activity. The analysis of the protein interaction with the ligand is an important element for drug delivery and molecular pathways information. The docking outcome within each ligand to the receptor was evaluated using the docking energy (Kcal/mol) as well as the binding of every ligand with active domains of α-glucosidase and α-amylase.
For the mushroom tyrosinase enzyme, rutin had the highest binding affinity (−8.9 kJ/mol), followed by scutellarin (−8.6 kJ/mol) and Lingstroside (−8.0 kJ/mol). The reference material (kojic acid) had a binding affinity of −5.3 kilojoules per mole. In contrast to ligands binding via traditional hydrogen bonding, those engaging via van der Waals forces and other weak intermolecular forces were discovered to have higher binding affinities. In the 2D docking data, van der Waals force interactions are substantially more prevalent than conventional hydrogen bonds between amino acids. This proved that our ligands have greater enzyme binding affinities than those previously reported.
4. Materials and Methods
4.1. Plant Collection and Extraction
S. glutinosus (whole plant) were collected from Abbottabad when plants were fully grown. S. glutinosus plant was identified by Dr. Sarwer from Islamia University, Bahawalpur, Pakistan and the specimen was placed in the Department of Botany’s Herbarium. The collected whole plant (08 kg) was extracted with 80% hydro alcoholic solvent (methanol and water with (80:20)) for 7 days with occasional shaking. The filtration was performed with filter paper and the solvent evaporation was conducted under vacuum through a rotary evaporator. The extract was fractionated with different solvents from low polarity to high polarity (n-hexane, chloroform, and n-butanol). The extracts are stored at the appropriate temperature (until required for further use).
4.2. Phytochemical Analysis
4.2.1. GC-MS Analysis
The equipment for GC-MS was Agilent, series 6890 and the detector was Hewlett Packard, 5973. Separations were attained by a coloumnHP-5MS column (length30 m × diameter 250 μm × thickness of film 0.25 μm). An electron ionization system with high energy electrons (70 eV) was utilized for spectroscopic detection by GC-MS. The temperature of the injector was 220 ± 0.2 °C and the transfer line 240 °C. The temperature of the oven was programmed from 60 °C to 246 °C at 3 °C/min. Pure helium gas was passed as a carrier at 1.02 mL/min at 210 °C. Prepared extracts, 1.0 µL diluted with methanol as a solvent, were injected at 250 °C in a split less method. The early temperature was positioned at 50–150 °C with a rising rate of 3 °C/min and held for 10 min. Finally, the temperature was amplified to 300 °C at a rate of 10 °C/min [33]. Detection was completed using a full scan mode between 35 to 600 m/z and with a gain factor of 5. The NIST 2011, Library was used for bioactive compounds identification.
4.2.2. LC-ESI-MS Analysis
Crude methanol extracts showing significantly increased antioxidant activities were also investigated using LC-ESI-MS-MS of model LTQ XL (Thermo Scientific, Waltham, MA, USA). The electron spray ionisation direct inject method was used for both negative and positive mode identification. The capillary temperature was kept constant at 290 °C. The voltage applied to the capillary was 4.7 kilovolts. We kept the sample flow rate constant at 7.8 μL/min. The 50–2000 m/z mass range was successfully controlled. The nature of the parent molecular ion dictated the energy range (5–30) over which collisions were induced for fragmentation during MS/MS. All of the samples came from the same place and under the same conditions. The ESI-MS/MS data were analysed by hand using specialised software (Xcalibur 2.0.7). The structure was elucidated using ChemDraw Ultra 12.0, and the results were correlated with previously published studies [34].
4.3. Antioxidant Activity
4.3.1. Radical Scavenging Activity
The DPPH and ABTS tests were used to assess the radical scavenging ability for extracts in accordance with the previously described procedure [35]. For the DPPH assay, whole-plant extract of 4 fractions each of 0.5 mL was added to 4 mL of DPPH (0.267 mM). The absorbance of different extracts was calculated at mg 517 nm. Trolox equivalents per gram of dry extract (mg TE/g extract) were used to calculate the findings. The ABTS assay was carried out by incubating the 2 mL of ABTS solution (2.5 mM), 0.5 mL of each extract solution, and 2.45 mM potassium persulfate (equal volume) for 30 min in the dark and at 734 nm absorbance was measured. Trolox equivalents per gram of dry extract (mg TE/g extract) were used to calculate the findings.
4.3.2. Reducing Power Assays
CUPRAC and FRAP assays have been used to determine the reductive potential of S. glutinosis whole-plant extracts, in accordance with previously described procedures [36]. For cupric ion reducing activity (CUPRAC assay), each extract (0.5 mL) was mixed with 10 mM CuCl2 (1 mL), 7.5 (mM) neocuproine (1 mL), and 1M NH4Ac buffer at pH 7.0 (1 mL). The absorbance was estimated at 450 nm after 30 min of incubation at room temperature. In the same manner, the blank sample was also prepared, other than the extract. The measurement unit was milligrams of Trolox equivalents per gram of dry extract (mg TE/g extract). For ferric-reducing antioxidant power (FRAP), 0.5 mL of extract solution in methanol (10 mg/10 mL) was vortexed with 2 mL of a FRAP reagent, and 225 µL of water was added and warmed at 37 °C. Then, the absorbance was read at 593 nm. The FRAP values were measured as milligrams of Trolox equivalents per gram of dry extract (mg TE/g extract).
4.3.3. Total Antioxidant Activity
The total antioxidant capacity of the extracts obtained from S. glutinosus was evaluated by using the phosphomolybdenum method in agreement with the previously described procedure [37], with few modifications. First, 0.5 mL of extract solutions with methanol (1 mg/1 mL) was added to reagent mixture consisting 0.6 M sulfuric acid (0.6 M), sodium phosphate (28 mM), and ammonium molybdate (4 mM). The mixture was incubated for 90 min at 95 °C and absorbances were recorded at 695 nm beside a blank sample having 0.5 mL methanol with a 3 mL reagent mixture. The measurement unit was milligrams of Trolox per gram of dry extract.
4.3.4. Metal Chelating Activity
The metal chelating assay was performed in accordance with the previously described procedure [17], with some modifications. The fraction solution with methanol (0.5 mL) was added to 0.05 mL FeCl2 (2 mM). The reaction was in progress, using 0.2 mL ferrozine (5 mM). Likewise, a blank sample was prepared without ferrozine. The absorbances of all fractions were recorded after incubation at room temperature for 10 min at 562 nm. The milligrams of EDTA equivalents per gram of dry extract (mg EDTAE/g extract) were used for measurement.
4.4. Enzyme Inhibitory Activities
The capacity of the different extract/fractions obtained from S. glutinosus to inhibit the α-amylase and α-glucosidase was described by the previously outlined procedure [17]. For α-amylase inhibition assay, the reaction mixture containing the different fractions of extract solution (0.5 mL) and alpha-amylase solution (10 μ/mL, 1 mL) with phosphate buffer (6 mM sodium chloride (pH 6.9) was put into the starch solution (0.05%, 0.5 mL). HCl (0.5 mL, 1 M) and 1 mL of iodine-potassium iodide solution were added to stop the reaction. The reaction mixtures were incubated for 10 min at 37 °C. The blank was prepared with the same procedure without the extract. The absorbance readings were noted at 630 nm. Milligrams of acarbose equivalents per gram of dry extract were the measuring unit.
The α-glucosidase inhibition assay was followed with the addition of 0.5 mL of different fraction solutions in equivalent concentrations of 0.5 mL glutathione (0.5 mg/mL) with α-glucosidase solution (0.2 u/mL) in phosphate buffer (pH 6.8) and PNPG (10 mM). After 15 min, the reaction was stopped with the addition of 0.5 mL of sodium carbonate solution (0.2 M). The absorbance readings were noted at 400 nm. The results were expressed in milligrams of acarbose equivalents per gram of dry extract (ACAEs/g extract). For tyrosinase inhibition, plant extracts were tested for their ability to inhibit the enzyme by the standard method already reported [38]. Kojic acid equivalents (KAE/g) were used to quantify the inhibitory effects on tyrosinase enzyme.
4.5. In Silico Analysis
Computer-aided molecular modeling was used to examine the conformational relationship between the compound and enzyme. The drug database was used for downloading the 3D structure of ligand molecules. Crystal structures of α-glucosidase (3F5L), α- amylase (3BC9), and tyrosinase (5M6B) were downloaded from the RCSB PDB protein data bank http://www.rcsb.org/pdb (accessed on 5 January 2022). The structure file (XML and PDB format) was converted to PDBQT format using Open Babel 2.4.1. AutoDock Vina, which was offered by the server, was used to determine the required hydrogen atoms. Auto grid software with connected grid data was employed for blind docking. The initial position, orientation, and torsions were all randomized (Santos et al., 2016). The energy and stability of the conformer were then minimized before docking to obtain the lowest energy and a more stable conformer. The extracted data were compared and validated with the experimental data for α-glucosidase and α-amylase complexed with acarbose ligand [39]. According to a recent study, most of the protein–ligand bindings are based on hydrogen bonds, ionic interactions, and van der Waals interactions. As a result, they were specifically targeted in this work by using Biovia/Discovery Studio 2021.
4.6. Statistical Analysis
The average of three similar experiments was used to calculate the effects, which were represented through the average ± SD of value. The results were analyzed using one-way ANOVA from SPSS v. 17.0. Statical significance was considered as the value of p < 0.05.
5. Conclusions
The present research has compared the biological properties and chemical characterization of different polarity solvent extract/fractions of S. glutinosus. The GC-MS analysis of chloroform and n-butanol fractions was performed and compared to provide more detail about the chemical profile. Fatty acids, phenols, monoterpenes, diterpenes, and sesquiterpenoids were identified as the key classes. In terms of inhibitory effects and in silico studies towards tyrosinase, α-amylase, and α-glucosidase, all extracts demonstrated different capabilities against these enzymes, and in silico studies of six selected compounds from GC-MS also provide the basis for ant-diabetic potential. Furthermore, molecular modelling of three flavonoids identified through LC-ESI-MS were docked to the tyrosinase enzyme to validate the plant’s tyrosinase inhibition potential. Based on our observation, S. glutinosus could be recognized as a promising potent biological agent possessing antioxidant, anti-diabetic, and anti-melanogenic properties. The high number of flavonoids and phenols identified in LC-ESI-MS analysis of the plant S. glutinosus in the present study may account for its powerful antioxidant and enzyme inhibition potential. However, further research concerning isolation, identification, and description of its bioactive compounds is essential to discover its potential applications in the field of medicine.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27206885/s1, Table S1: List of abbreviations; Table S2: Pharmacological properties of various species of the genus Strobilanthes [40,41,42,43,44,45,46,47,48,49,50].
Author Contributions
Conceptualization, M.A., U.K. and H.S.; methodology, S.A., U.K., G.A.M.M. and M.A.; software, N.J., U.K. and M.M.I.; validation, M.A.A., I.P. and J.B.; formal analysis, H.S.; investigation, U.K.; resources, S.A. and Z.M.E.-B.; data curation, S.K. and A.H.L.; writing—original draft preparation, M.A., U.K. and H.S.; writing—review and editing, U.K., F.S.A., A.A.S.A. and H.S.; project administration N.J., A.H.L. and I.P.; funding acquisition G.A.M.M., M.M.I. and Z.M.E.-B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The authors acknowledge the financial support of Taif University Researchers Supporting Project number (TURSP-2020/14), Taif University, Taif, Saudi Arabia.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zahra W., Rai S.N., Birla H., Singh S.S., Rathore A.S., Dilnashin H., Keswani C., Singh S.P. Bioeconomy for Sustainable Development. Springer; Singapore: 2020. Economic Importance of Medicinal Plants in Asian Countries; pp. 359–377. [DOI] [Google Scholar]
- 2.Jamshidi-Kia F., Lorigooini Z., Amini-Khoei H. Medicinal plants: Past history and future perspective. J. Herbmed Pharmacol. 2018;7:1–7. doi: 10.15171/jhp.2018.01. [DOI] [Google Scholar]
- 3.Silverman R.B., Holladay M.W. The Organic Chemistry of Drug Design and Drug Action. Academic Press; Cambridge, MA, USA: 2014. [DOI] [Google Scholar]
- 4.Williams J.T., Ahmad Z. Priorities for Medicinal Plants Research and Development in Pakistan. Medicinal and Aromatic Plants Program in Asia (MAP PA); New Delhi, India: 1999. [Google Scholar]
- 5.Newman D.J., Cragg G.M., Snader K.M. The influence of natural products upon drug discovery. Nat. Prod. Rep. 2000;17:215–234. doi: 10.1039/a902202c. [DOI] [PubMed] [Google Scholar]
- 6.Adomako-Bonsu A.G., Chan S.L.F., Pratten M., Fry J.R. Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: Importance of physico-chemical characteristics. Toxicol. Vitr. 2017;40:248–255. doi: 10.1016/j.tiv.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Behravan E., Razavi B.M., Hosseinzadeh H. Review of Plants and Their Constituents in the Therapy of Cerebral Ischemia. Phytother. Res. 2014;28:1265–1274. doi: 10.1002/ptr.5187. [DOI] [PubMed] [Google Scholar]
- 8.Moylan E.C., Bennett J.R., Carine M.A., Olmstead R.G., Scotland R.W. Phylogenetic relationships among Strobilanthes sl (Acanthaceae): Evidence from ITS nrDNA, trnL-F cpDNA, and morphology. Am. J. Bot. 2004;91:724–735. doi: 10.3732/ajb.91.5.724. [DOI] [PubMed] [Google Scholar]
- 9.Terao H. Ph.D. Thesis. Kyoto University; Kyoto, Japan: 1983. Taxonomic Study of the Genus Strobilanthes Blume (Acanthaceae): Generic Delimitation and Infrageneric Classification. [Google Scholar]
- 10.Ajaib M., Ishtiaq M., Shafi F., Bhatti K.H., Zahid M.T. Antimicrobial and antioxidant analysis of Strobilanthes glutinous: An unexplored medicinal plant. Biosci. Res. 2020;17:1521–1534. [Google Scholar]
- 11.Nguyen H.P., Schug K.A. The advantages of ESI-MS detection in conjunction with HILIC mode separations: Fundamentals and applications. J. Sep. Sci. 2008;31:1465–1480. doi: 10.1002/jssc.200700630. [DOI] [PubMed] [Google Scholar]
- 12.Lin D., Xiao M., Zhao J., Li Z., Xing B., Li X., Kong M., Li L., Zhang Q., Liu Y., et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules. 2016;21:1374. doi: 10.3390/molecules21101374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siddique H.R., Saleem M. Beneficial health effects of lupeol triterpene: A review of preclinical studies. Life Sci. 2011;88:285–293. doi: 10.1016/j.lfs.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 14.Yu F.R., Lian X.Z., Guo H.Y., McGuire P.M., Li R.D., Wang R., Yu F.H. Isolation and characterization of methyl esters and derivatives from Euphorbia kansui (Euphorbiaceae) and their inhibitory effects on the human SGC-7901 cells. J. Pharm. Pharm. Sci. 2005;8:528–535. [PubMed] [Google Scholar]
- 15.Banjarnahor S.D.S., Artanti N. Antioxidant properties of flavonoids. Med. J. Indones. 2014;23:239–244. doi: 10.13181/mji.v23i4.1015. [DOI] [Google Scholar]
- 16.Foti M.C. Antioxidant properties of phenols. J. Pharm. Pharmacol. 2007;59:1673–1685. doi: 10.1211/jpp.59.12.0010. [DOI] [PubMed] [Google Scholar]
- 17.Khurshid U., Ahmad S., Saleem H., Nawaz H.A., Zengin G., Locatelli M., Mahomoodally M.F., Abidin S.A.Z., Tousif M.I., Ahemad N. Phytochemical composition and in vitro pharmacological investigations of Neurada procumbens L. (Neuradaceae): A multidirectional approach for industrial products. Ind. Crop. Prod. 2019;142:111861. doi: 10.1016/j.indcrop.2019.111861. [DOI] [Google Scholar]
- 18.Pervaiz I., Saleem H., Sarfraz M., Tousif M.I., Khurshid U., Ahmad S., Zengin G., Sinan K.I., Locatelli M., Mahomoodally F.M., et al. Multidirectional insights into the phytochemical, biological, and multivariate analysis of the famine food plant (Calligonum polygonoides L.).: A novel source of bioactive phytocompounds. Food Res. Int. 2020;137:109606. doi: 10.1016/j.foodres.2020.109606. [DOI] [PubMed] [Google Scholar]
- 19.Adegbola P.I., Adetutu A., Olaniyi T.D. Antioxidant activity of Amaranthus species from the Amaranthaceae family—A review. S. Afr. J. Bot. 2020;133:111–117. doi: 10.1016/j.sajb.2020.07.003. [DOI] [Google Scholar]
- 20.Jiang M.-Z. In vitro and in vivo studies of antioxidant activities of flavonoids from Adiantum capillus-veneris L. Afr. J. Pharm. Pharmacol. 2011;5:2079–2085. doi: 10.5897/ajpp11.500. [DOI] [Google Scholar]
- 21.Nimse S.B., Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5:27986–28006. doi: 10.1039/C4RA13315C. [DOI] [Google Scholar]
- 22.Khan S., Nazir M., Raiz N., Saleem M., Zengin G., Fazal G., Mukhtar M., Tousif M.I., Tareen R.B., Abdallah H.H., et al. Phytochemical profiling, in vitro biological properties and in silico studies on Caragana ambigua stocks (Fabaceae): A comprehensive approach. Ind. Crop. Prod. 2019;131:117–124. doi: 10.1016/j.indcrop.2019.01.044. [DOI] [Google Scholar]
- 23.Khokhar S., Apenten R.O. Iron binding characteristics of phenolic compounds: Some tentative structure–activity relations. Food Chem. 2003;81:133–140. doi: 10.1016/S0308-8146(02)00394-1. [DOI] [Google Scholar]
- 24.Yanishlieva N.V., Marinova E.M., Gordon M.H., Raneva V.G. Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chem. 1999;64:59–66. doi: 10.1016/S0308-8146(98)00086-7. [DOI] [Google Scholar]
- 25.Tchimene M.K., Nwaehujor C.O., Ezenwali M., Okoli C.C., Iwu M.M. Free radical scavenging activity of lupeol isolated from the methanol leaf extract of Crateva adansonii Oliv. (Capparidaceae) Int. J. Pharmacogn. Phytochem. Res. 2016;8:419–426. [Google Scholar]
- 26.Owen R., Mier W., Giacosa A., Hull W., Spiegelhalder B., Bartsch H. Phenolic compounds and squalene in olive oils: The concentration and antioxidant potential of total phenols, simple phenols, secoiridoids, lignansand squalene. Food Chem. Toxicol. 2000;38:647–659. doi: 10.1016/S0278-6915(00)00061-2. [DOI] [PubMed] [Google Scholar]
- 27.Campbell R.K. Type 2 diabetes: Where we are today: An overview of disease burden, current treatments, and treatment strategies. J. Am. Pharm. Assoc. 2009;49:S3–S9. doi: 10.1331/JAPhA.2009.09077. [DOI] [PubMed] [Google Scholar]
- 28.Etxeberria U., de la Garza A.L., Campión J., Martínez J.A., I Milagro F. Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin. Ther. Targets. 2012;16:269–297. doi: 10.1517/14728222.2012.664134. [DOI] [PubMed] [Google Scholar]
- 29.Joseph S., Kumar L., Bai V.N. Evaluation of anti-diabetic activity of Strobilanthes cuspidata in alloxan induced diabetic rats and the effect of bioactive compounds on inhibition of [alpha]-amylase enzyme. J. Pharmacogn. Phytochem. 2016;5:169. [Google Scholar]
- 30.Muddathir A., Yamauchi K., Batubara I., Mohieldin E., Mitsunaga T. Anti-tyrosinase, total phenolic content and antioxidant activity of selected Sudanese medicinal plants. S. Afr. J. Bot. 2017;109:9–15. doi: 10.1016/j.sajb.2016.12.013. [DOI] [Google Scholar]
- 31.Jdey A., Falleh H., Ben Jannet S., Hammi K.M., Dauvergne X., Ksouri R., Magné C. Phytochemical investigation and antioxidant, antibacterial and anti-tyrosinase performances of six medicinal halophytes. S. Afr. J. Bot. 2017;112:508–514. doi: 10.1016/j.sajb.2017.05.016. [DOI] [Google Scholar]
- 32.Mocan A., Zengin G., Uysal A., Gunes E., Mollica A., Degirmenci N.S., Alpsoy L., Aktumsek A. Biological and chemical insights of Morina persica L.: A source of bioactive compounds with multifunctional properties. J. Funct. Foods. 2016;25:94–109. doi: 10.1016/j.jff.2016.05.010. [DOI] [Google Scholar]
- 33.Hayat M.M., Uzair M. Biological potential and GC-MS analysis of phytochemicals of Farsetia hamiltonii (Royle) Biomed. Res. 2019;30:609–616. doi: 10.35841/biomedicalresearch.30-19-241. [DOI] [Google Scholar]
- 34.Qamar M., Akhtar S., Ismail T., Sestili P., Tawab A., Ahmed N. Anticancer and anti-inflammatory perspectives of Pakistan’s indigenous berry Grewia asiatica Linn (Phalsa) J. Berry Res. 2020;10:115–131. doi: 10.3233/JBR-190459. [DOI] [Google Scholar]
- 35.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 36.Zengin G., Nithiyanantham S., Locatelli M., Ceylan R., Uysal S., Aktumsek A., Selvi P.K., Maskovic P. Screening of in vitro antioxidant and enzyme inhibitory activities of different extracts from two uninvestigated wild plants: Centranthus longiflorus subsp. longiflorus and Cerinthe minor subsp. auriculata. Eur. J. Integr. Med. 2016;8:286–292. doi: 10.1016/j.eujim.2015.12.004. [DOI] [Google Scholar]
- 37.Berk S., Tepe B., Arslan S., Sarikurkcu C. Screening of the antioxidant, antimicrobial and DNA damage protection potentials of the aqueous extract of Asplenium ceterach DC. Afr. J. Biotechnol. 2011;10:8902–8908. [Google Scholar]
- 38.Grochowski D.M., Uysal S., Aktumsek A., Granica S., Zengin G., Ceylan R., Locatelli M., Tomczyk M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017;20:365–372. doi: 10.1016/j.phytol.2017.03.005. [DOI] [Google Scholar]
- 39.Kitamura M., Okuyama M., Tanzawa F., Mori H., Kitago Y., Watanabe N., Kimura A., Tanaka I., Yao M. Structural and Functional Analysis of a Glycoside Hydrolase Family 97 Enzyme from Bacteroides thetaiotaomicron. J. Biol. Chem. 2008;283:36328–36337. doi: 10.1074/jbc.M806115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ho Y.-L. Evaluation of antinociceptive, anti-inflammatory and antipyretic effects of Strobilanthes cusia leaf extract in male mice and rats. Am. J. Chin. Med. 2003;31:61–69. doi: 10.1142/S0192415X03000783. [DOI] [PubMed] [Google Scholar]
- 41.Mitscher L.A., Baker W. Tuberculosis: A search for novel therapy starting with natural products. Med. Res. Rev. 1998;18:363–374. doi: 10.1002/(SICI)1098-1128(199811)18:6<363::AID-MED1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 42.Tsai Y.-C. Antiviral action of tryptanthrin isolated from Strobilanthes cusia leaf against human coronavirus NL63. Biomolecules. 2020;10:366. doi: 10.3390/biom10030366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agarwal R., Rangari V. Antiinflammatory and antiarthritic activities of lupeol and 19 alpha-H lupeol isolated from Strobilanthus callosus and Strobilanthus ixiocephala roots. Indian J. Pharmacol. 2003;35:384–387. [Google Scholar]
- 44.Kumar S. Acute and chronic inflammation studies of Strobilanthes callosus leaves extract on rat model. Inflammopharmacology. 2013;21:233–239. doi: 10.1007/s10787-012-0150-8. [DOI] [PubMed] [Google Scholar]
- 45.Singh B., Sahu P., Sharma M. Anti-inflammatory and antimicrobial activities of triterpenoids from Strobilanthes callosus Nees. Phytomedicine. 2002;9:2355–2359. doi: 10.1078/0944-7113-00143. [DOI] [PubMed] [Google Scholar]
- 46.Abou Muamar A.F.H. Master’s Thesis. Universiti Putra Malaysia; Serdang, Malaysia: 1999. Isolation, Identification and Evaluation of Antibacterial Activity of the Semi-purified Compound from Strobilanthes crispus (L. Bremek) [Google Scholar]
- 47.Bakar M.F.A. Master’s Thesis. Universiti Putra Malaysia; Serdang, Malaysia: 2005. Effects of Strobilanthes crispus Crude and Tea Extracts in Streptozotocin-Induced Hyperglycemic Rats. [Google Scholar]
- 48.Fadzelly A., Asmah R., Fauziah O. Effects of Strobilanthes crispus tea aqueous extracts on glucose and lipid profile in normal and streptozotocin-induced hyperglycemic rats. Plant Foods Hum. Nutr. 2006;61:6–11. doi: 10.1007/s11130-006-0002-z. [DOI] [PubMed] [Google Scholar]
- 49.Ghasemzadeh A., Jaafar H.Z., Rahmat A. Phytochemical constituents and biological activities of different extracts of Strobilanthes crispus (L.) Bremek leaves grown in different locations of Malaysia. BMC Complement. Altern. Med. 2015;15:1–10. doi: 10.1186/s12906-015-0873-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balasubramaniam G., Sekar M., Varadarajan M., Badami S. Antioxidant and hepatoprotective activities of Strobilanthes kunthianus against carbon tetrachloride-induced hepatotoxicity in rats. Pharmacogn. J. 2020;12:1143–1151. doi: 10.5530/pj.2020.12.161. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.