Table 1. Optimization of the Reaction Conditionsa.
| entry | solvent | 2a (mmol) | T (°C) | yieldb (%) |
|---|---|---|---|---|
| 1 | DMF | 0.2 | 90 | 55 |
| 2 | DMSO | 0.2 | 90 | 35 |
| 3 | EtOH | 0.2 | 90 | 27 |
| 4 | Acetone | 0.2 | 90 | 49 |
| 5 | EA | 0.2 | 90 | 31 |
| 6 | DCE | 0.2 | 90 | 24 |
| 7 | CH3CN | 0.2 | 90 | 82 |
| 8 | 1,4-dioxane | 0.2 | 90 | 57 |
| 9 | Toluene | 0.2 | 90 | 63 |
| 10 | THF | 0.2 | 90 | 50 |
| 11 | n-hexane | 0.2 | 90 | 48 |
| 12 | CH3CN | 0.2 | 70 | 68 |
| 13 | CH3CN | 0.2 | 110 | 81 |
| 14 | CH3CN | 0.3 | 90 | 86 |
| 15 | CH3CN | 0.4 | 90 | 93 |
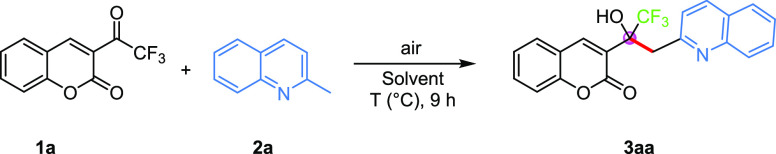
All reactions were performed with 1a (0.2 mmol) and 2a in solvent (2.0 mL) under an air atmosphere at 90 °C for 9 h.
Isolated yield. DMF = N,N-dimethylformamide, DMSO = dimethylsulfoxide, EA = ethyl acetate, DCE = 1,2-dichloroethane, and THF = tetrahydrofuran.