Abstract
Background
Remdesivir is the only antiviral agent approved for the treatment of hospitalized coronavirus disease 2019 (COVID-19) patients requiring supplemental oxygen. Studies show conflicting results regarding its effect on mortality.
Methods
In this single center observational study, we included adult hospitalized COVID-19 patients. Patients who were treated with remdesivir were compared to controls. Remdesivir was administered for 5 days. To adjust for any imbalances in our cohort, a propensity score matched analysis was performed. The aim of our study was to analyze the effect of remdesivir on in-hospital mortality and length of stay (LOS).
Results
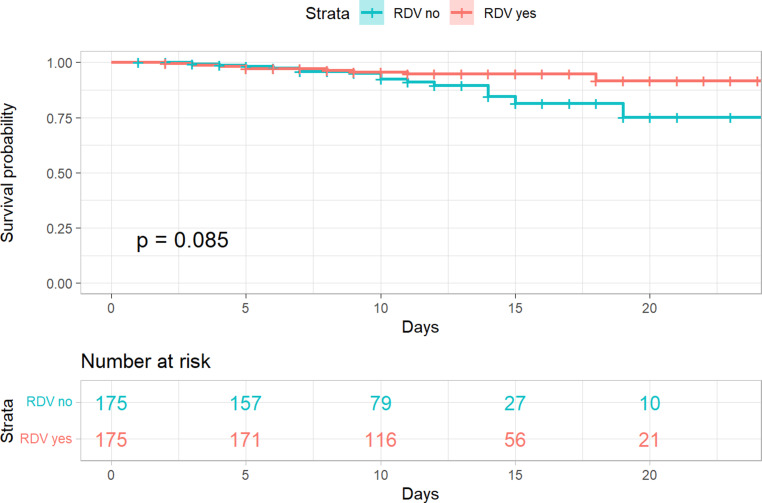
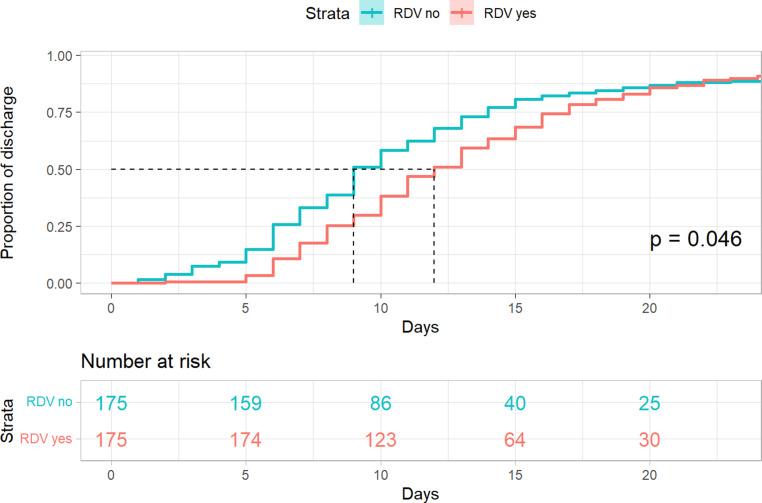
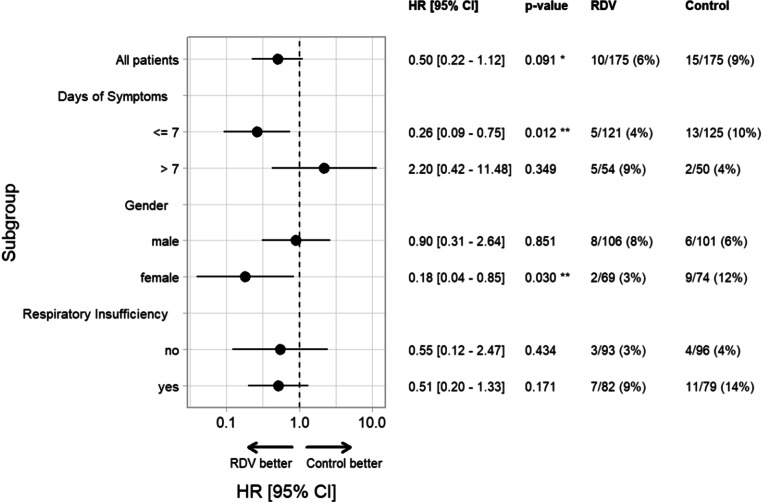
After propensity score matching, 350 patients (175 remdesivir, 175 controls) were included in our analysis. Overall, in-hospital mortality was not significantly different between groups remdesivir 5.7% [10/175] vs. control 8.6% [15/175], hazard ratio 0.50, 95% confidence interval (CI) 0.22–1.12, p = 0.091. Subgroup analysis showed a significant reduction of in-hospital mortality in patients who were treated with remdesivir ≤ 7 days of symptom onset remdesivir 4.2% [5/121] vs. control 10.4% [13/125], hazard ratio 0.26, 95% CI 0.09 to 0.75, p = 0.012 and in female patients remdesivir 2.9% [2/69] vs. control 12.2% [9/74], hazard ratio 0.18 95%CI 0.04 to 0.85, p = 0.03. Patients in the remdesivir group had a significantly longer LOS (11 days vs. 9 days, p = 0.046).
Conclusion
Remdesivir did not reduce in-hospital mortality in our whole propensity score matched cohort, but subgroup analysis showed a significant mortality reduction in female patients and in patients treated within ≤ 7 days of symptom onset. Remdesivir may reduce mortality in patients who are treated in the early stages of illness.
Supplementary Information
The online version of this article (10.1007/s00508-022-02098-9) contains supplementary material, which is available to authorized users.
Keywords: In-hospital mortality, Austria, Within 7 days, Gender differences, Length of stay
Introduction
The RNA polymerase inhibitor remdesivir is an adenosine analogue with broad antiviral activity against various respiratory viruses (e.g. RSV), human coronaviruses (severe acute respiratory syndrome coronavirus 1, Middle East respiratory syndrome–related coronavirus, severe acute respiratory syndrome coronavirus 2) and causes of viral hemorrhagic fevers (e.g. Ebola virus and Marburg virus) [1, 2]. The prodrug is rapidly converted intracellularly into its active nucleoside triphosphate metabolite GS-443902 and incorporated into the viral RNA which leads to chain termination and inhibition of viral replication [1]. While other in vitro active agents like hydroxychloroquine and lopinavir/ritonavir failed to show clinical benefit in hospitalized COVID-19 patients in large randomized controlled trials [3–5], remdesivir was the first antiviral drug approved for the treatment of COVID-19.
In the ACTT‑1 trial, a multicenter double blind RCT involving 1062 patients, treatment with intravenous remdesivir for up to 10 days reduced median time to clinical recovery from 15 to 10 days and led to a numerically lower 29-day mortality. The effects were mainly driven by patients receiving low flow oxygen [6].
In the recently updated results from the multinational, open label, randomized platform SOLIDARTY trial (n = 8275) remdesivir reduced 28-day mortality in patients requiring oxygen but were not mechanically ventilated [30]. In a meta-analysis of 4 published RCTs remdesivir did not reduce 28-day mortality overall, but the subgroup of patients receiving low-flow oxygen at the time of randomization showed a significant reduction in 28-day mortality [7]. Further remdesivir may increase the proportion of patients who recover and reduce progression to mechanical ventilation and ECMO, while the results on effects on length of stay are conflicting [6–8].
Observational trials add additional evidence to assess the efficacy of new drugs in the real-world-setting. In our observational single center study, we performed a propensity score matched analysis to investigate the effect of remdesivir on in-hospital mortality and length of stay in hospitalized COVID-19 patients.
Methods
Study design and data collection
This retrospective propensity score matched study was conducted at the Department for Infectious Diseases and Tropical Medicine at the Klinik Favoriten in Vienna, Austria. The department consists of 2 normal wards with 28 beds each and an intensive care unit (ICU) with 10 beds. Each normal ward is equipped with at least 5 high-flow oxygen beds. High-flow oxygen was administered via the AIRVO2 (Fisher & Paykel healthcare limited, Auckland, New Zealand) and initiated by treating physicians based on clinical parameters.
Patients ≥ 18 years with polymerase chain reaction confirmed SARS-CoV‑2 infection who were directly admitted to our normal ward (non-ICU ward) were eligible for the study. Remdesivir was administered intravenously for 5 days with a loading dose of 200 mg on day 1 and 100 mg on days 2–5. If patients were discharged before day 5 of hospitalization, treatment was discontinued on the day of discharge. Remdesivir was not administered in an outpatient setting. Contraindications for remdesivir administration included an estimated glomerular filtration rate below 30 ml/min and elevated liver enzymes of more than 5 times the upper normal limit. The decision whether to administer remdesivir was made by the treating physicians and was based on clinical judgement. After remdesivir was approved for the treatment of hospitalized patients, it was used at our department primarily in patients with low-flow oxygen and before symptom day 10. During the pandemic our local prescribing practice changed quickly and patients earlier in the course of disease without need for oxygen support received remdesivir as well. Remdesivir was not initiated when patients where already on high-flow oxygen therapy. If a patient on remdesivir deteriorated and high-flow oxygen or mechanical ventilation was necessary, the treatment course of 5 days was completed.
Patient symptoms, medical history, laboratory parameters and complications were collected via a standardized form during hospital admission. Incomplete data were updated retrospectively from patient electronic health records whenever possible. Data was collected from 6 June 2020 to 6 March 2021. The study was approved by the ethics committee of the capital city of Vienna.
Concomitant medication
All patients received low molecular weight heparin (enoxaparin or nadroparin) as thromboprophylaxis. If patients had any indications for full anticoagulation before admission and were on direct anticoagulants, these drugs continued to be administered during their hospital stay. All patients needing oxygen support received 6 mg dexamethasone for up to 10 days. No patient received tocilizumab.
Definition of variables and outcome parameters
The first day of any perceived COVID-19 associated symptom (e.g., headache, cough, sore throat, fever, dyspnea) was considered to be the disease onset. Fever was defined as a body temperature ≥ 38 °C measured either by the patient at home (using any kind of thermometer) or during a medical examination (via ear thermometers). Respiratory insufficiency was defined as SpO2 ≤ 93% without supplementary oxygen. The primary outcome of the study was in-hospital mortality and length of hospital stay.
Statistical analyses
Results were expressed as relative frequencies for categorical variables, mean with standard deviations (SD) for continuous variables and median with interquartile range for skewed distributions. The χ2-test was used for categorial variables while t-test and Mann-Whitney‑U test were used for non-skewed and skewed continuous variables, respectively. Kaplan-Meier plots were calculated for time-event analysis and the log-rank test was used to test for significance. Hazard ratios were calculated with Cox regressions and the Wald test was used to test for significance. P‑values of < 0.05 were considered to be statistically significant. All statistical analyses were performed using R [9].
To account for the observational character and the imbalances of our data we used the propensity score (PS) [10]. In a first step we defined the following variables as relevant for the calculation of the PS: Gender, age, duration of symptoms before admission (measured in days), number of comorbidities, respiratory insufficiency on admission (dichotomous) as well as C‑reactive protein (CRP). Only baseline characteristics were used for the PS. Our unmatched original data contained 588 rows providing 1 patient per row. After omitting data rows containing missing values among those variables, we retained 536 observations for further analyses. Different models of propensity score matching were calculated using the R package “MatchIt” [11]. The balances of the model covariates after matching were each compared by estimating the standardized mean difference (SMD), which is commonly used to assess the balance of covariate distribution of treatment groups as the comparison between different units of measurements is allowed [12]. A value below 0.25 (Stuart) and a stricter cut-off below 0.1 (Austin)indicate a good balance [13]. The stricter cut-off was used for our analysis.
The nearest neighbor matching with and without caliper and genetic matching models did not generate a full balanced data set with SMDs of > 0.1 in some variables. The best match for our data was achieved with the genetic matching with the caliper model (caliper 0.1). With this model we could generate a full balanced dataset (SMD < 0.1) for all covariates which includes 350 observations (175 treated with remdesivir, 175 controls). Visualizations of PS distributions and SMD across the different models can be viewed in the supplemental figure S1.
Results
Unmatched cohort
The unmatched cohort consisted of 588 patients hospitalized for COVID-19 of whom 227 received remdesivir. Baseline characteristics of the patients were unbalanced. Patients in the remdesivir group were significantly older (65.2 years vs. 57.2 years, p < 0.001) and had a higher rate of hypertension (59.5% vs. 43.8%, p < 0.001), diabetes mellitus (30.4% vs. 18%, p < 0.001) and chronic obstructive lung disease (13.2% vs. 6.6%, p < 0.001).
On admission, patients in the remdesivir group had a higher rate of respiratory insufficiency (55.9% vs. 29.4%, p < 0.001) and were admitted to the hospital earlier in the course of their disease (6 days vs. 7 days after symptom onset, p < 0.001). Furthermore, patients in this group had a higher CRP (60 mg/l vs. 33.1 mg/l, p < 0.001) and lower lymphocyte count (0.79 G/l vs. 1.07 G/l, p < 0.001) on admission. See Tables 1 and 2 for details.
Table 1.
Baseline characteristics on admission
| Unmatched cohort | Propensity score matched cohort | |||||
|---|---|---|---|---|---|---|
| Control (n = 361) |
Remdesivir (n = 227) |
p-value | Control (n = 175) |
Remdesivir (n = 175) |
p-value | |
| Age (mean, SD) | 57.6 years (19.9) | 65.2 years (14.8) | < 0.001 | 63.9 years (17.2) | 63.3 years (15.0) | 0.733 |
| Gender | ||||||
|
Female Male |
173 (47.9%) 188 (52.1%) |
90 (39.6%) 137 (60.4%) |
0.060 |
74 (42.3%) 101 (57.7%) |
69 (39.4%) 106 (60.6%) |
0.664 |
|
Body mass index (Md, IQR) |
27.4 (24.4–31.1) n = 273 |
27.8 (24.9–33.5) n = 178 |
0.159 |
27.4 (24.8–30.8) n = 129 |
27.3 (24.5–32.9) n = 142 |
0.726 |
| Number of comorbidities (mean, SD) | 1.05 [1.27] | 1.52 [1.37] | < 0.001 | 1.35 [1.26] | 1.41 [1.33] | 0.712 |
| Hypertension | 158 (43.8%) | 135 (59.5%) | < 0.001 | 97 (55.4%) | 99 (56.6%) | 0.914 |
| Diabetes mellitus | 65 (18.0%) | 69 (30.4%) | < 0.001 | 41 (23.4%) | 49 (28.0%) | 0.392 |
| Chronic heart failure | 15 (4.2%) | 11 (4.8%) | 0.849 | 8 (4.6%) | 7 (4.0%) | > 0.999 |
| Chronic obstructive lung disease | 24 (6.6%) | 30 (13.2%) | 0.011 | 14 (8.0%) | 20 (11.4%) | 0.367 |
| Chronic kidney disease | 43 (11.9%) | 35 (15.4%) | 0.273 | 28 (16.0%) | 23 (13.1%) | 0.545 |
| Atrial fibrillation | 33 (9.1%) | 21 (9.3%) | > 0.999 | 17 (9.7%) | 17 (9.7%) | > 0.999 |
| Days of symptom onset before admission (Md, IQR) |
7.0 [3.0–9.5] |
6.0 [4.0–8.0] |
0.005 |
6.0 [3.0–8.0] |
6.0 [4.0–8.0] |
0.626 |
| Respiratory insufficiency | 106 (29.4%) | 127 (55.9%) | < 0.001 | 79 (45.1%) | 82 (46.9%) | 0.830 |
The following variables were used to calculate the propensity-score: gender, age, duration of symptoms before admission (measured in days), number of comorbidities, respiratory insufficiency on admission (dichotomous), C‑reactive protein
SD standard deviation, Md median, IQR interquartile range
Table 2.
Laboratory parameters on admission
| Unmatched cohort | Propensity score matched cohort | |||||
|---|---|---|---|---|---|---|
| Control (n = 361) |
Remdesivir (n = 227) |
p-value | Control (n = 175) |
Remdesivir (n = 175) |
p-value | |
|
Leucocytes in G/l (Md, IQR) |
5.6 G/l (4.5–7.6) n = 341 |
5.9 G/l (4.6–8.1) n = 225 |
0.121 |
5.6 G/l (4.5–8.0) n = 173 |
5.9 (4.4–7.7) n = 175 |
0.360 |
|
Lymphocytes in G/l (Md, IQR) |
1.07 G/l (0.74–1.53) n = 323 |
0.79 G/l (0.57–1.18) n = 209 |
0.001 |
0.93 G/l (0.67–1.27) n = 164 |
0.79G/l (0.57–1.19) n = 161 |
0.095 |
|
C‑reactive protein in mg/l (Md, IQR) |
33.1 mg/l (10.1–73.4) n = 333 |
60.0 mg/l (27.2, 110) n = 225 |
< 0.001 |
53.3 mg/l (20.2–93.9) n = 175 |
56.2 mg/l (23.8–104) n = 175 |
0.454 |
|
Ferritin in μ/l (Md, IQR) |
357 μ/l (158–712) n = 297 |
607 μ/l (289–1020) N = 192 |
< 0.001 |
495 μ/l (259–865) n = 155 |
618 μ/l (315–1010) n = 150 |
0.244 |
|
D‑dimer in mg/l (Md, IQR) |
0.73 mg/l (0.43–1.19) n = 287 |
0.83 mg/l (0.54–1.41) n = 206 |
< 0.001 |
0.78 mg/l (0.52–1.21) n = 149 |
0.76 mg/l (0.48–1.31) n = 160 |
0.414 |
The following variables were used to calculate the propensity-score: gender, age, duration of symptoms before admission (measured in days), number of comorbidities, respiratory insufficiency on admission (dichotomous), C‑reactive-protein
Md median, IQR interquartile range
During hospitalization, patients in the remdesivir group received dexamethasone more frequently (73.1% vs. 38.8%, p < 0.001). Patients in the remdesivir group were significantly more often treated with high-flow oxygen (41.4% vs. 10.2%, p < 0.001) and transferred to the ICU (12.3% vs. 3%, p < 0.001).
In-hospital mortality was not different between groups (remdesivir 7.5% vs. control 7.2%, p = 1), but patients in the remdesivir group had a significantly longer length of stay (13 days vs. 7 days, p < 0.001). For further details see Table 3.
Table 3.
Course of disease and outcome
| Unmatched cohort | Propensity score matched cohort | |||||
|---|---|---|---|---|---|---|
| Control (n = 361) |
Remdesivir (n = 227) |
p-value | Control (n = 175) |
Remdesivir (n = 175) |
p-value | |
| Dexamethasone | 140 (38.8%) | 166 (73.1%) | < 0.001 | 100 (57.1%) | 123 (70.3%) | 0.014 |
| High-flow oxygen | 37 (10.2%) | 94 (41.4%) | < 0.001 | 29 (16.6%) | 71 (40.6%) | < 0.001 |
| ICU admission | 11 (3.0%) | 28 (12.3%) | < 0.001 | 11 (6.3%) | 18 (10.3%) | 0.245 |
| In-hospital mortality | 26 (7.2%) | 17 (7.5%) | 1.0 | 15 (8.6%) | 10 (5.7%) | 0.085a |
| Length of stay in days (Md, IQR) |
7.0 days (4.0–11.0) |
13.0 days (8.25–17.)] |
< 0.001 |
9.0 days (6.00–13.0) |
11.0 days (8.0–16.0) |
0.046a |
The following variables were used to calculate the propensity score: gender, age, duration of symptoms before admission (measured in days), number of comorbidities, respiratory insufficiency on admission (dichotomous), C‑reactive protein
ICU intensive care unit, Md median, IQR interquartile range
ap‑value derived from log-rank test
More than 90% of patients who were treated with remdesivir received the full 5‑day course.
Propensity score matched cohort
In total 350 patients were propensity score matched. Baseline characteristics were well balanced between groups. No differences were observed with regards to gender, age, body mass index, comorbidities, days of symptoms before admission, laboratory parameters or respiratory insufficiency on admission.
Patients in the remdesivir group received dexamethasone more frequently during the course of the disease (70.3% vs. 57.1%, p = 0.014). More patients in the remdesivir group were treated with high-flow oxygen (40.6% vs. 16.6%, p < 0.001). See Tables 1, 2 and 3.
In-hospital mortality was not statistically different between groups (log rank p = 0.085), but a trend towards lower mortality in patients treated with remdesivir was observed (remdesivir 5.7% [10/175] vs. control 8.6% [15/175], hazard-ratio 0.50 95% CI 0.22 to 1.12, p = 0.091). Patients in the remdesivir group had a significantly longer length of stay (11 days vs. 9 days, p = 0.046). The corresponding Kaplan-Meier-plots are shown in Figs. 1 and 2.
Fig. 1.
Kaplan-Meier plot—In-hospital mortality—propensity score matched cohort. The following variables were used to calculate the propensity score: gender, age, duration of symptoms before admission (measured in days), number of comorbidities, respiratory insufficiency on admission (dichotomous), C‑reactive protein. RDV remdesivir
Fig. 2.
Kaplan-Meier plot—Length of stay—propensity score matched cohort. The following variables were used to calculate the propensity score: gender, age, duration of symptoms before admission (measured in days), number of comorbidities, respiratory insufficiency on admission (dichotomous), C‑reactive protein. RDV remdesivir
Subgroup analysis showed a significant reduction of in-hospital mortality in patients treated with remdesivir ≤ 7 days of symptom onset (remdesivir 4.2% [5/121] vs. control 10.4% [13/125], hazard ratio 0.26 95% CI 0.09 to 0.75, p = 0.012) and in female patients (remdesivir 2.9% [2/69] vs. control 12.2% [9/74], hazard ratio 0.18 95% CI 0.04 to 0.85, p = 0.03). Remdesivir did not reduce mortality in the subgroup of patients who were treated > 7 days of symptom onset, in male patients or in patients with and without respiratory insufficiency on admission. For details see Fig. 3.
Fig. 3.
Subgroup analysis—In-hospital mortality—propensity score matched cohort. The following variables were used to calculate the propensity score: gender, age, duration of symptoms before admission (measured in days), number of comorbidities, respiratory insufficiency on admission (dichotomous), C‑reactive-protein. RDV remdesivir, HR hazard ratio
A subgroup analysis of patients ≤ 65 years and > 65 years could not be performed because no deaths occurred in the younger age group.
Discussion
In our propensity score matched cohort remdesivir did not reduce in-hospital mortality, but in the subgroup of patients who received remdesivir ≤ 7 days of symptom onset and in female patients there was a significant reduction in in-hospital mortality. Furthermore, patients treated with remdesivir were, on average, in hospital for 2 days longer.
Large RCTs failed to show any significant effect of remdesivir on mortality in the overall population [4, 6], which corresponds with our results. Subgroup analysis of the ACTT‑1 trial showed a benefit in 29-day mortality in patients who needed low-flow oxygen at time of randomization and received remdesivir [6].
A lower 28-day mortality (14.6% vs. 16.3%, number needed to treat, NNT = 58) was observed in the subgroup of patients who required oxygen but were not mechanically ventilated in the recently updated SOLIDARITY trial [30]. Unfortunately, patients who were receiving low-flow and high-flow oxygen at the time of randomization were not analyzed separately [30]. A meta-analysis of 4 RCTs did show an absolute mortality reduction of 2.4% (9.7% vs. 12.1%, NNT = 42) in the subgroup of patients with supplemental oxygen and no need for mechanical ventilation [7]. The same was shown in a metanalysis when the updated results of the SOLIDARITY trial were included (13.4% vs. 15.4%, NNT = 50) [30]. A significant mortality reduction was also shown in observational trials [8]. The absolute reduction of in-hospital mortality was 2.9% in our study, but this was not statistically significant. In a propensity score matched analysis (N = 1767) remdesivir significantly reduced the 28-day mortality only in patients receiving low-flow oxygen at baseline [14]. These findings could not be reproduced in our study, where the need for oxygen support at time of treatment initiation did not have any impact on the effect of remdesivir on mortality in the matched cohort. Very recently, in a large propensity score matched analysis with approximately 60,000 patients a significant 14-day and 28-day mortality reduction was shown in patients treated with remdesivir. The absolute mortality reduction was approximately 3–5% and demonstrated in the overall population, as well as in patients with low-flow oxygen and with no oxygen support [15].
In the multicenter RCT DISCOVERY trial (N = 857) remdesivir reduced neither 28-day mortality overall nor 28-day mortality in the subgroup of patients with low-flow oxygen (approximately two thirds received low-flow oxygen). Patients were randomized a median 9 days after onset of symptoms, which might have attenuated any potential benefit of remdesivir [16]. A large retrospective study featuring 2607 hospitalized patients with low-flow oxygen support, of whom 438 received remdesivir, demonstrated a significant mortality reduction in patients receiving remdesivir within ≤ 6 days of symptom onset. The effect was even more pronounced in patients who were treated within 3 days of becoming symptomatic [17]. In line with these results, we could show that remdesivir significantly reduced the in-hospital mortality of patients who were treated within ≤ 7 days of symptom onset. As respiratory deterioration usually develops in the second week after symptom onset, this finding corresponds with the benefit of remdesivir in patients with low flow oxygen described in several studies mentioned above [6, 7, 14]. These findings were also observed in a supplemental analysis of the ACTT‑1 trial in which the strongest effect of remdesivir on time to recovery was seen in patients treated within ≤ 6 days of symptom onset [6]; however, such an effect was not demonstrated in the DISCOVERY trial [16]. In both trials the effect of the timing of remdesivir on mortality was not analyzed [6, 16]. Unfortunately, information regarding days since symptom onset is missing from the SOLIDARITY trial [30].
We therefore suggest that early administration of remdesivir might be crucial in reducing COVID-19 mortality. Furthermore, differences in the timing of drug administration might explain the differences in mortality rates benefit between patients with low-flow oxygen vs. patients with high-flow oxygen or mechanical ventilation in other studies. The concept that early administration can be a key factor in antiviral efficacy has already been proven by studying other viral infections like influenza [18–20]. Very recently, the oral antiviral COVID-19 drugs molnupiravir and nirmatrelvir/ritonavir have been shown to reduce the coprimary endpoint hospitalization and death in outpatients at risk when treated within 5 days of symptom onset [27, 28]. The same has been shown for outpatients treated with a 3-day course of remdesivir [29], which further supports this concept.
In our study, treatment with remdesivir reduced in-hospital mortality in female patients. This might be a coincidental finding because some other larger trials did not demonstrate any gender-related treatment effect of remdesivir [6, 16]; however, gender-specific analyses were not performed in all studies [4, 7, 17]. Gender differences in drug metabolism and efficacy have been described for various drug and differences in body weight, volume of distribution, drug-drug interactions or differences in transporter or enzyme expression may contribute to clinical efficacy [21–24].
More patients in the remdesivir group were treated with high-flow oxygen and dexamethasone. This might suggest that patients in the remdesivir group had a more severe disease trajectory and that this was the reason why remdesivir was started in the first place. A residual confounding may be likely even after the propensity score matching. If this is true, the effect of remdesivir may have been underestimated in our analysis. In the entire cohort, patients who received remdesivir were generally older and had more comorbidities, which further supports this hypothesis. An alternative explanation could be that remdesivir actually does not reduce, and may even increase, the number of patients who require high-flow oxygen and admission to the ICU; however, this seems unlikely because in several previous studies remdesivir reduced the progression to mechanical ventilation and ECMO [6–8, 16, 26].
Dexamethasone has shown to reduce mortality in COVID-19 patients requiring supplemental oxygen [25]. As more patients in our remdesivir cohort received dexamethasone this might have influenced our results and overestimated the effect of remdesivir treatment.
Interestingly, patients who were treated with remdesivir had a longer hospitalization. More than 90% of patients received the full 5‑day course of remdesivir and this may have prolonged their length of stay.
Our study has several limitations. It was a single center open-label observational trial with unbalanced baseline characteristics between groups. The indication to prescribe remdesivir was not standardized and varied by the treating physician. We did not include patients who were directly admitted to the ICU, only patients who were admitted to our normal ward initially were included in the study. We did not differentiate between patients who needed different levels of low-flow oxygen when remdesivir was initiated. Further data about the dominant SARS-CoV‑2 subtype are not available which might have influenced our results. The strength of our study is that it is a real-life cohort which reflects common practice in the clinical management of hospitalized COVID-19 patients. Furthermore, time since symptom onset was clearly documented and early administration vs. late administration could therefore be analyzed. We were able to perform a propensity score matched analysis with a strict cut-off criterion of SMD < 0.1 to adjust for any imbalances in the non-matched cohort. Unfortunately, a potential residual confounding still exists after the propensity score matching. Another strength of our study is that it is the first one that could show the benefit of remdesivir in a population of patients living in Austria.
Studies suggest reduced hospitalization and mortality rates in patients infected with omicron variants [31, 32]. Remdesivir shows activity in omicron SARS-CoV‑2 variants in vitro with similar inhibitory concentrations like in the older variants [33]. Further studies are needed to investigate if any clinical benefit can be achieved by treatment with remdesivir in patients infected with omicron variants.
In summary, no significant difference in in-hospital mortality in patients treated with remdesivir could be observed. Subgroup analysis showed a mortality reduction in female patients and in patients treated within ≤ 7 days of symptom onset. The question regarding whether treatment with remdesivir should be restricted to patients with low-flow oxygen, or extended to all hospitalized patients admitted shortly after symptom onset remains unanswered. Patients who received remdesivir had a longer hospitalization, most likely due to the fixed 5‑day treatment course which was completed by almost every patient.
In conclusion, early treatment with remdesivir may reduce mortality in hospitalized COVID-19 patients. A 5-day course of remdesivir may be considered in patients admitted to hospital during the first 7 days after symptom onset with no or low-flow oxygen support.
Supplementary Information
Figure S1: Standard mean differences of all propensity score matched models
Acknowledgments
Acknowledgements
Data was collected as a part of the diploma theses of the authors SA, LE, SJ and AK.
Funding
This work did not receive any funding.
Author Contribution
MK, EP, SO and AK had the idea of the study. MK wrote the manuscript. LK analyzed the data. FK supervised the data analysis. EP, TS and WH proof-read the manuscript. SA, LE, SJ, AK, SO, MP, DT and MT collected the data. HL, AA, CW and AZ supervised the study.
Declarations
Conflict of interest
M. Karolyi, L. Kaltenegger, E. Pawelka, A. Kuran, M. Platzer, D. Totschnig, F. Koenig, W. Hoepler, H. Laferl, S. Omid, T. Seitz, M. Traugott, S. Arthofer, L. Erlbeck, S. Jaeger, A. Kettenbach, A. Assinger, C. Wenisch and A. Zoufaly declare that they have no competing interests.
Ethical standards
The study was approved by the ethics committee of the capital city of Vienna. Consent for publication: not applicable.
Footnotes
Availability of data and material
Data will be made available if requested.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aleissa MM, Silverman EA, Paredes Acosta LM, Nutt CT, Richterman A, Marty FM. New perspectives on antimicrobial agents: Remdesivir treatment for COVID-19. antimicrob Agents Chemother. 2020;65(1):e01814–e01820. doi: 10.1128/AAC.01814-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulangu S, Dodd LE, Davey RT, Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381(24):2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amani B, Khanijahani A, Amani B. Hydroxychloroquine plus standard of care compared with standard of care alone in COVID-19: a meta-analysis of randomized controlled trials. Sci Rep. 2021;11(1):11974. doi: 10.1038/s41598-021-91089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Solidarity Trial Consortium. Pan H, Peto R, et al. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med. 2020 doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.RECOVERY Collaborative Group Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020;396(10259):1345–1352. doi: 10.1016/S0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaka AS, MacDonald R, Greer N, et al. Major update: Remdesivir for adults with COVID-19 : a living systematic review and meta-analysis for the American college of physicians practice points. Ann Intern Med. 2021;174(5):663–672. doi: 10.7326/M20-8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rezagholizadeh A, Khiali S, Sarbakhsh P, Entezari-Maleki T. Remdesivir for treatment of COVID-19; an updated systematic review and meta-analysis. Eur J Pharmacol. 2021;897:173926. doi: 10.1016/j.ejphar.2021.173926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.R Core Team . R: A language and environment for statistical computing. 2020. [Google Scholar]
- 10.Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med. 2008;27(12):2037–2049. doi: 10.1002/sim.3150. [DOI] [PubMed] [Google Scholar]
- 11.Ho DE, Imai K, King GEAS. Matchit: nonparametric preprocessing for parametric causal inference. J Stat Soft. 2011;42(8):1–28. doi: 10.18637/jss.v042.i08. [DOI] [Google Scholar]
- 12.Zhang Z, Kim HJ, Lonjon G, Zhu Y, AME Big-Data Clinical Trial Collaborative Group Balance diagnostics after propensity score matching. Ann Transl Med. 2019;7(1):16. doi: 10.21037/atm.2018.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao QY, Luo JC, Su Y, Zhang YJ, Tu GW, Luo Z. Propensity score matching with R: conventional methods and new features. Ann Transl Med. 2021;9(9):812. doi: 10.21037/atm-20-3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olender SA, Walunas TL, Martinez E, et al. Remdesivir versus standard-of-care for severe Coronavirus disease 2019 infection: an analysis of 28-day mortality. Open Forum Infect Dis. 2021;8(7):ofab278. doi: 10.1093/ofid/ofab278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mozaffari E, Chandak A, Zhang Z, et al. Remdesivir treatment in hospitalized patients with COVID-19: a comparative analysis of in-hospital all-cause mortality in a large multi-center observational cohort. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ader F, Bouscambert-Duchamp M, Hites M, et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. Lancet Infect Dis. 2021;3099(21):00485–00480. doi: 10.1016/S1473-3099(21)00485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021 doi: 10.1093/jac/dkab321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bassetti M, Castaldo N, Carnelutti A. Neuraminidase inhibitors as a strategy for influenza treatment: pros, cons and future perspectives. Expert Opin Pharmacother. 2019;20(14):1711–1718. doi: 10.1080/14656566.2019.1626824. [DOI] [PubMed] [Google Scholar]
- 19.Muthuri SG, Venkatesan S, Myles PR, et al. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med. 2014;2(5):395–404. doi: 10.1016/S2213-2600(14)70041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doll MK, Winters N, Boikos C, et al. Safety and effectiveness of neuraminidase inhibitors for influenza treatment, prophylaxis, and outbreak control: a systematic review of systematic reviews and/or meta-analyses. J Antimicrob Chemother. 2017;72(11):2990–3007. doi: 10.1093/jac/dkx271. [DOI] [PubMed] [Google Scholar]
- 21.Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48(3):143–157. doi: 10.2165/00003088-200948030-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valodara AM, Johar SR K. Sexual dimorphism in drug metabolism and pharmacokinetics. Curr Drug Metab. 2019;20(14):1154–1166. doi: 10.2174/1389200220666191021094906. [DOI] [PubMed] [Google Scholar]
- 23.Umeh OC, Currier JS, Park JG, Cramer Y, Hermes AE, Fletcher CV. Sex differences in lopinavir and ritonavir pharmacokinetics among HIV-infected women and men. J Clin Pharmacol. 2011;51(12):1665–1673. doi: 10.1177/0091270010388650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karolyi M, Omid S, Pawelka E, et al. High dose lopinavir/Ritonavir does not lead to sufficient plasma levels to inhibit SARS-coV-2 in hospitalized patients with COVID-19. Front Pharmacol. 2021;12:704767. doi: 10.3389/fphar.2021.704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.RECOVERY Collaborative Group. Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of Remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324(11):1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2021 doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammond J, Leister-Tebbe H, Gardner A, et al. Oral Nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022 doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gottlieb RL, Vaca CE, Paredes R, et al. Early Remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2021 doi: 10.1056/NEJMoa2116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO Solidarity Trial Consortium. Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO solidarity randomised trial and updated meta-analyses. Lancet. 2022;399(10339):1941–1953. doi: 10.1016/S0140-6736(22)00519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ward IL, Bermingham C, Ayoubkhani D, et al. Risk of covid-19 related deaths for SARS-CoV-2 omicron (B.1.1.529) compared with delta (B.1.617.2): retrospective cohort study. BMJ. 2022;378:e070695. doi: 10.1136/bmj-2022-070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takashita E, Kinoshita N, Yamayoshi S, et al. Efficacy of antibodies and antiviral drugs against Covid-19 omicron variant. n Engl J Med. 2022;386(10):995–998. doi: 10.1056/NEJMc2119407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Standard mean differences of all propensity score matched models