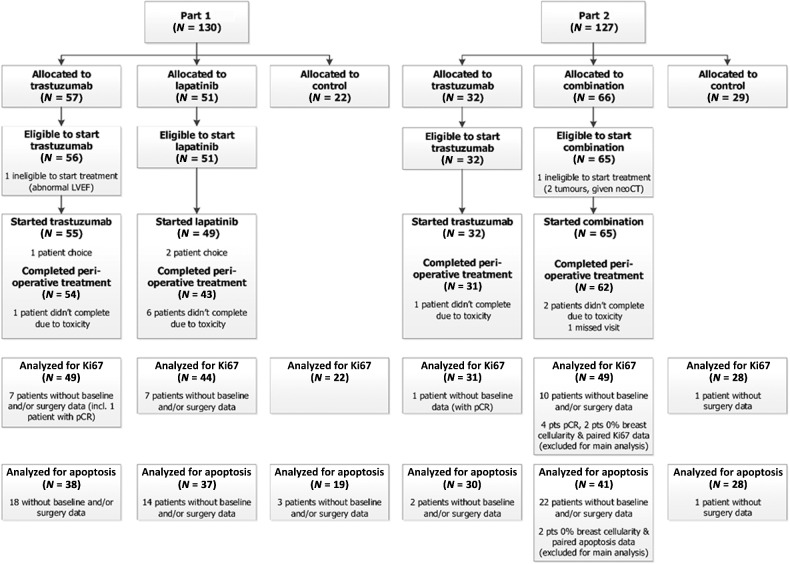
Figure 1.
The CONSORT diagram summarizes patients recruited into each part of the trial, patients randomized, patients eligible to start treatment, patients who started treatment, and those who completed perioperative treatment as per protocol. In part 1, 22 patients were allocated to control, 57 to trastuzumab, and 51 to lapatinib; in part 2, 29 were allocated to control, 32 to trastuzumab, and 66 to the combination. Overall, 255 (99%) patients were considered eligible to start treatment and included in the analysis of perioperative endpoints. Of the 204 patients in the treatment groups, 201 patients (99%) received some perioperative treatment, with 190/201 (95%) completing the 11 days of perioperative treatment. The figure also describes how many patients available for analysis of coprimary endpoints Ki67 and apoptosis. Only patients with both paired samples and enough tumor tissue for biomarker analysis were included in the analysis: 223 patients (88%) had paired Ki67 data and 193 (76%) had paired apoptosis data available for analysis. Patients with pCR or 0% breast cellularity were excluded from main analysis of Ki67 and apoptosis.