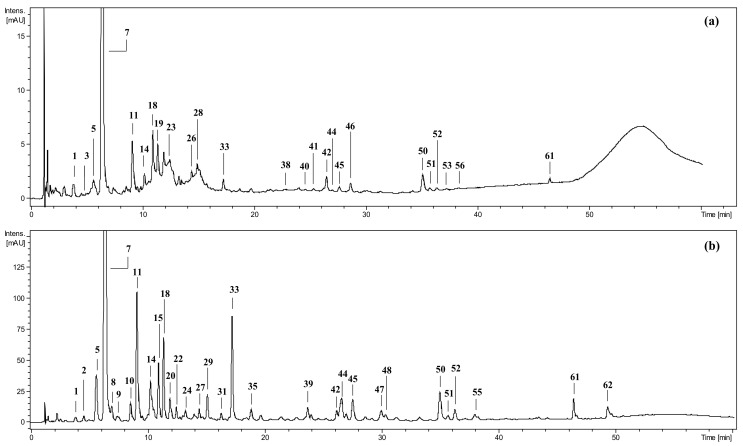
Figure 1.
Example UHPLC chromatograms at 280 nm of (a) methanol–water (75:25, v/v) extract from fresh P. spinosa fruits, MEF; and (b) its ethyl acetate fraction, EAFF. Peak identification: 1—vanillic acid O-hexoside; 2—protocatechuic acid; 3—unidentified; 5—cis-3-O-caffeoylquinic acid; 7—neochlorogenic acid; 8—p-hydroxybenzoic acid; 9—neochlorogenic acid hexoside; 10—vanilloyl malate hexoside; 11—3-O-p-coumaroylquinic acid; 14—chlorogenic acid; 15—cis-3-O-feruloylquinic acid; 18—cryptochlorogenic acid; 19—cyanidin 3-O-glucoside; 20—caffeic acid 3/4-O-hexoside; 22—3-O-feruloylquinic acid; 23—cyanidin 3-O-rutinoside; 24—vanillin; 26—peonidin 3-O-glucoside; 27—cis-3-O-p-coumaroylquinic acid; 28—peonidin 3-O-rutinoside; 29—4-O-caffeoylshikimic acid; 31—4-O-feruloylquinic acid; 33, 35—caffeoylshikimic acid; 38—kaempferol dihexoside; 39—p-coumaroylshikimic acid; 40—quercetin hexoside-pentoside; 41—quercetin rhamnoside-hexoside; 42—hyperoside; 44—rutin; 45—isoquercitrin; 46—quercetin 3-O-(2″-O-β-D-glucopyranosyl)-α-L-arabinofuranoside; 47—reinutrin; 48—guaiaverin; 50—avicularin; 51—multinoside A; 52—quercitrin; 53—isorhamnetin rhamnoside-hexoside; 55—quercetin malyl-pentoside; 56—quercetin acetyl-hexoside; 61—quercetin acetyl-hexoside-rhamoside; 62—quercetin. Nomenclature of pseudodepsides according to IUPAC. For details of compounds’ identification and quantification see Magiera et al. [11].