Figure.
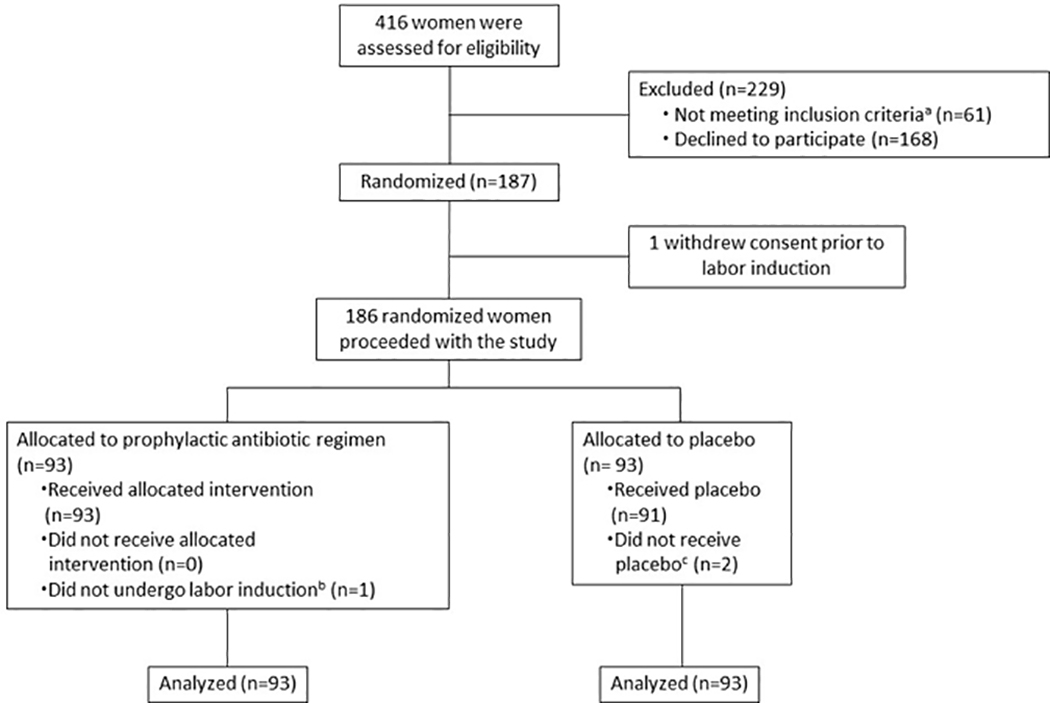
CONSORT (Consolidated Standards of Reporting Trials) enrollment flow diagram. This diagram shows the number of patients approached for the study, the number who declined, the number who were enrolled and randomized, and the number of women in each treatment group.
a30 had an allergy to one or more of the study medications, 8 underwent pre-labor cesarean delivery, 6 had a major fetal anomaly, 5 were minor patients without parent consent, 5 had infection requiring antibiotic therapy, 3 had ruptured membranes >12 hours, 3 were preterm, and 1 was incarcerated
bOne participant presented for induction of labor and received study drug, but over the course of her admission was found to be in spontaneous labor and did not undergo labor induction
cTwo participants allocated to the placebo group did not receive the placebo: One participant was randomized but ultimately did not undergo labor induction (delivered via primary cesarean due to concern for fetal macrosomia); one participant was excluded after randomization due to discovery of an infection requiring antibiotic treatment (chlamydia) prior to the start of her labor induction
