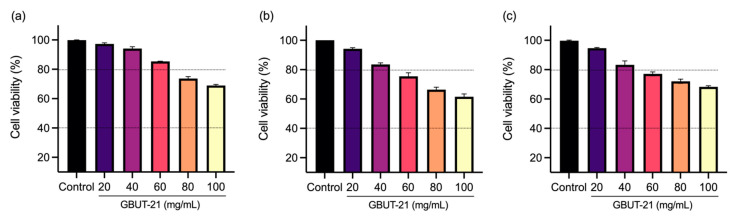
Figure 2.
The therapeutic concentration of GBUT11 CSF is well-tolerated by human cell lines. MTT assay was performed on HeLa, HEK, and HCE cell lines to check the percentage viability in the presence of the sample. Cells were seeded in 96-well plates at a density of 4 × 105 and treated with the sample for 24 h. Percentage viability: (a) HEK, (b) HeLa, and (c) HCE cells at different concentrations of the sample.