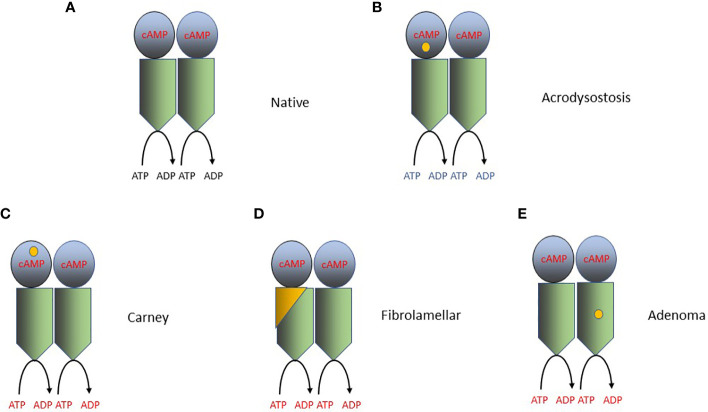
Figure 2.
Mutations that activate PKA in cancer and other disorders. PKA catalyzes the phosphorylation of serine and/or threonine amino acids located at specific sites in its protein substrates. ATP is the phosphodonor and therefore the reaction converts ATP to ADP. The PKA holoenzyme is a tetramer of 2 catalytic subunits (C-subunits, Cα or Cβ; blue in the figure), and 2 regulatory subunits (R-subunits, RIα, RIβ RIIα or RIIβ; green in the figure). The RIα subunit is encoded by PRKARA1A and the Cα or Cβ subunits are encoded by PRKACA and PRKACB, respectively. Each R subunit forms a homodimer through the interaction of helices at its amino terminus and also interacts with at least one C subunit. cAMP binds to a specific domain located within each R subunit and produces a conformational change in the PKA holoenzyme, increasing its catalytic (protein kinase) activity. The location of the specific driver mutations in PKA implicated in oncogenesis are shown in each panel by a yellow dot. (A) The native (wild-type) PKA holoenzyme. (B) Mutations producing acrodysostosis. These mutations are localized to the second cAMP-binding domain of RIα, where they perturb the switch between the active (cAMP-bound) and inactive conformations of PKA. The mutations produce cAMP-resistant PKA holoenzymes and thereby reduce PKA activity. (C) Mutations producing Carney Complex. Mutations in one of several amino acids in RIα reduce the stability of RIα or its interaction with Cα/β and therefore increase PKA action. (D) Mutations in fibrolamellar hepatocellular carcinoma. The DNAJB1-PRKACA fusion (yellow and green) is capable of binding to RIα and RIIα subunits, creating in each case a functional holoenzyme. DNAJB1-PRKACA holoenzymes are more abundant in cells, producing increased PKA enzymatic activity. (E) Mutations in adrenal adenomas. Mutations in the Cα subunit, such as L205R, alters the ability of the Cα subunits to be regulated by the R subunits, leading to constitutive, cAMP-independent signaling. The L205R and W196R mutations also appear to exclude the mutant holoenzymes from their AKAP anchors, allowing them to diffuse indiscriminately throughout the cell, producing phosphorylation of non-physiologic PKA substrates.